Psychedelic

Psychedelics (also known as serotonergic hallucinogens) are a class of psychoactive substances that produce an altered state of consciousness marked by unusual changes in perception, mood, and cognitive processes.[1]
While the precise mechanism of action is not known, psychedelic substances are thought to produce their effects by binding to serotonin (5-hydroxytryptamine or 5-HT) receptors in the central nervous system, particularly the 5-HT2a subtype. Serotonin plays a number of critical roles throughout the human body and is a key neurotransmitter involved in the functioning and regulation of sensory perception, behavior, mood, cognition and memory.[2]
Human usage of psychedelics predates written history, and there is growing evidence that they were employed by early cultures in a variety of sociocultural and ritual contexts.[1] In modern times, psychedelic substances are used for a number of purposes that span from the traditional shamanic forms (such as the use of ayahuasca in the Amazon jungle, or peyote among Native Americans) to more modern forms of New Age spiritual, transpersonal, or religious practices.
Psychedelics, particularly those in the traditional or herbal forms, are sometimes referred to as entheogens (i.e. "generating the divine within")[3] by those who use them for these purposes, although they are also used in modern recreational settings.
Subjective effects can vary significantly depending on the subclass, but generally include some form of open and closed-eye visuals, time distortion, enhanced introspection, conceptual thinking, euphoria, and ego loss. The so-called classical psychedelics, which consist of LSD, psilocybin mushrooms, mescaline, and DMT (ayahuasca) are considered to produce the archetypal psychedelic effects and also have the most established safety profiles.
Psychedelics can be divided into three major sub-classes: tryptamines, lysergamides, and phenethylamines. Psychedelic tryptamines (e.g. psilocybin, 4-AcO-DMT) are either based on or derived from dimethyltryptamine (DMT), lysergamides (e.g. LSA, AL-LAD) from LSD, and phenethylamines (e.g. 2C-B, DOM) from mescaline.
Unlike other highly prohibited substances, most psychedelics have not been shown to be physiologically toxic and none have been shown to be addictive.[1] However, adverse psychological reactions such as severe anxiety, paranoia, delusions, and psychosis are always possible, particularly for those predisposed to mental disorders.[4]
As a result, it is highly advised to use harm reduction practices if using these substances.
Etymology
The term "psychedelic" was coined by psychiatrist Humphry Osmond in 1956 as an alternative descriptor for hallucinogens in the context of psychedelic psychotherapy.[5]
Seeking a name for the experience induced by LSD, Osmond contacted Aldous Huxley, a personal acquaintance and advocate for the therapeutic use of the substance. Huxley coined the term "phanerothyme," from the Greek terms for "manifest" (φανερός) and "spirit" (θύμος).
In a letter to Osmond, he wrote:
To make this mundane world sublime,
Take half a gram of phanerothyme
To which Osmond responded:
To fathom Hell or soar angelic,
Just take a pinch of psychedelic[6]
"Psychedelic" derives from the Greek words ψυχή (psyche, "soul, mind") and δηλείν (delein, "to manifest") which taken together mean "mind-manifesting" or "soul-manifesting." The implication was that psychedelics can allow one to access the soul and develop unused potentials of the human mind.[7][8]
It was on this term that Osmond eventually settled, because it was "clear, euphonious and uncontaminated by other associations."[9] This mongrel spelling of the word "psychedelic" was loathed by American ethnobotanist Richard Evans Schultes, but championed by Timothy Leary, who thought it sounded better.[10]
Due to the expanded use of the term "psychedelic" in pop culture and a perceived incorrect verbal formulation, Carl A.P. Ruck, Jeremy Bigwood, Danny Staples, Jonathan Ott, and R. Gordon Wasson later proposed the term "entheogen" to describe the religious or spiritual experience produced by such substances.[11]
Method of action
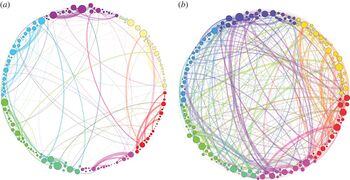
The width of the links is proportional to their weight and the size of the nodes is proportional to their strength. Note that the proportion of heavy links between communities is much higher (and very different) in the psilocybin group, suggesting greater integration[12]
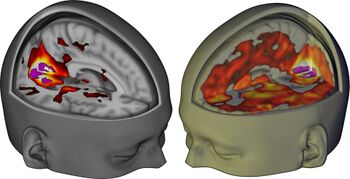
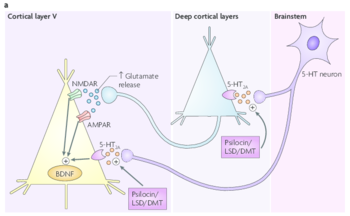
This glutamate release leads to an activation of AMPA and NMDA receptors on cortical pyramidal neurons. in addition, hallucinogens directly activate 5-HT2A receptors located on cortical pyramidal neurons. This activation is thought to ultimately lead to increased expression of brain-derived neurotrophic factor (BDNF).[14]
Psychedelics act on serotonin receptors (also referred to as 5-HT receptors) via the way in which they act as full or partial agonists through their structural similarity to the serotonin molecule. It has a higher affinity than serotonin itself for the receptors, therefore preventing serotonin from binding to the receptors by competing with it.
While the method of action behind psychedelics is not fully understood, serotonergic psychedelics are known to show affinities for various 5-HT receptors and may be classified by their activity at different 5-HT subsites, such as 5-HT1A, 5-HT1B, 5-HT2A, etc.
Many serotonergic psychedelics share very close chemical and structural similarities to serotonin itself. There is a consensus that serotonergic psychedelics produce their effects by acting as uniquely effective partial agonists at 5-HT2A receptor sites.[1]
Subjective effects
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Visual effects 
-
Enhancements
-
Distortions
-
Geometry
-
Hallucinatory states
Cognitive effects 
-
Enhancements
- Analysis enhancement
- Emotion enhancement
- Catharsis
- Creativity enhancement
- Dream potentiation
- Empathy, affection, and sociability enhancement
- Immersion enhancement
- Increased music appreciation
- Increased sense of humor
- Multiple thought streams
- Novelty enhancement
- Personal meaning enhancement
- Rejuvenation
- Thought acceleration
- Thought connectivity
-
Suppressions
-
Alterations
-
Transpersonal states
Physical effects 
-
Enhancements
-
Suppression
-
Alterations
-
Uncomfortable effects
Auditory effects 
Multisensory effects 
Chemical classes
The "classical psychedelics" are all classed as serotonergic in nature.[1] This means that they structurally mimic the endogenous neurotransmitter known as serotonin, the neurotransmitter that regulates higher-level brain functions such as mood, sensory perception, cognition, and memory.[2]
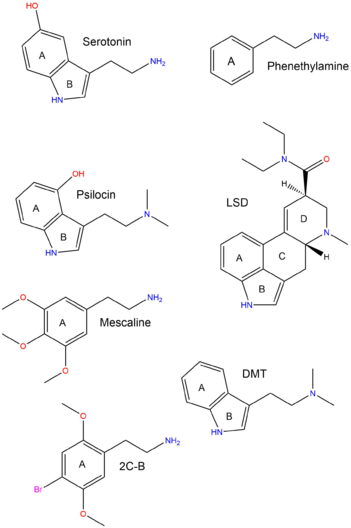
The diagram to the right shows the structural similarities and differences between the various classes of psychedelics and the serotonin neurotransmitter. The three classes (phenethylamines, lysergamides and tryptamines) all contain the same chemical rings (which have been labeled).
- A represents the benzene ring, which all three classes contain.
- B represents the pyrrole ring in both tryptamines and lysergamides.
- A and B together form the indole ring.
- C (cyclohexane) and D are only contained in the lysergamides, possibly contributing to their potency.
Examples
Tryptamines
-
Base tryptamines
-
Substituted tryptamines
- 2-Me-DET
- 2-Me-DMT
- 4-AcO-DET (Ethacetin)
- 4-AcO-DiPT (Ipracetin)
- 4-AcO-DMT (Psilacetin)
- 4-AcO-DPT (Pracetin)
- 4-AcO-EPT (Epracetin)
- 4-AcO-MET (Metacetin)
- 4-AcO-MiPT (Mipracetin)
- 4-AcO-MPT (Mepracetin)
- 4-HO-αMT
- 4-HO-DET (Ethocin)
- 4-HO-DiPT (Iprocin)
- 4-HO-DMT (Psilocin)
- 4-HO-DPT (Procin)
- 4-HO-EPT (Eprocin)
- 4-HO-MCPT
- 4-HO-MET (Metocin)
- 4-HO-MiPT (Miprocin)
- 4-HO-MPT (Meprocin)
- 4-HO-PiPT
- 4-Me-αMT
- 4-Me-αET
- 4-MeO-DMT
- 4-MeO-MiPT
- 5-Pro-DMT
- 5-Br-DMT
- 5-Chloro-DMT
- 5-Chloro-αMT
- 5-Eto-DMT
- 5-Fluoro-αMT
- 5-Fluoro-DMT
- 5-HO-DMT (Bufotenin)
- 5,6-MeO-MiPT
- 5-MeO-7-TMT
- 5-MeO-AET
- 5-MeO-aMT
- 5-MeO-BFE (Dimemebfe)
- 5-MeO-DALT (Foxtrot)
- 5-MeO-DiBF
- 5-MeO-DiPT (Foxy)
- 5-MeO-DPT
- 5-MeO-DMT
- 5-MeO-MALT
- 5-MeO-MET
- 5-MeO-MiPT (Moxy)
- 5-MeO-NMT
- 5-MeO-PiPT
- 5-MeO-pyr-T
- 5-MeO-Tryptamine
- 5-MeS-DMT
- 5-MeO-TMT
- 6-MeO-THH
- 7-Me-αET
- 7-Me-DMT
- Alpha,N-DMT
- Ibogaine
- Melatonin
Toxicity and harm potential
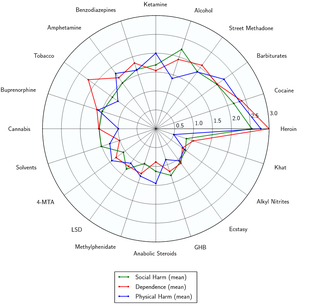
While more research is needed, most psychedelics (and especially classical psychedelics) appear to be physiologically well-tolerated and have very low toxicity relative to dose.[1] Most psychedelics have very few physical side effects associated with acute exposure.
Various studies have shown that, in reasonable doses in a sufficiently prepared context, they are unlikely to present negative physical, cognitive, psychiatric or other toxic consequences. There is no evidence that classical psychedelics cause damage to any human body organ.[17]
However, it should be noted that some exceptions exist, such as some members of the 25x-NBOMe, 2C-T-x, DOx and 5-MeO series. Some substances of the NBOMe family, particularly 25I-NBOMe, have been associated with fatal overdoses.[18][19][20]
However, while psychedelics are generally not capable of causing direct bodily harm or death, their use can still have serious negative consequences. For example, they are capable of impairing the judgment and attention of the user which may cause erratic or high-risk behaviors. In extreme cases, the user may fall under the delusion that they are a character in a dream or physically invincible which may cause them to jump off of a building or run into a busy road.[1]
Additionally, intense negative experiences and psychotic episodes ("bad trips") can cause psychological trauma if not properly managed or treated. This is particularly a concern in non-supervised settings or when heavy doses are used.
Psychosis
Psychedelics may trigger or exacerbate symptoms (e.g. delusions, mania, psychosis) in those who have or are predisposed to mental illness such as bipolar disorder or schizophrenia.[17] Those with a personal or family history of mental illness (including anxiety and depression) should not use LSD without the advice of a qualified medical professional.
Lethal dosage
Unlike many other illicit substances, psychedelics typically do not have established lethal dosages. There are no well-documented deaths attributable to the direct pharmacological action of any psychedelic, with the notable exception of the 25x-NBOMe, 2C-T-x, and 5-MeO series.
Dependence and abuse potential
Psychedelics are considered to have low abuse potential.[17] There are no literature reports of successful attempts to train animals to self-administer psychedelics — an animal model predictive of abuse liability — indicating that it does not have the necessary pharmacology to either initiate or maintain dependence.[17] Likewise, there is no human clinical evidence that psychedelics cause addiction. Finally, there is virtually no withdrawal syndrome when chronic use of these substances is stopped.[21]
Tolerance to the effects of most psychedelics builds almost immediately after ingestion and hits a peak once the effects wear off. After that, it takes about 5-7 days for the tolerance to be reduced to half and 1-2 weeks to be back at baseline (in the absence of further consumption). Most psychedelics present cross tolerance with all psychedelics, meaning they will have a reduced effect.
Notable exceptions to this include DMT and related base tryptamines like DPT and MET, which are thought to produce little to no tolerance or cross-tolerance.
Another exception includes psychedelic phenethylamines like 2C-B. While the exact mechanism is not understood, generally tolerance is thought to rise immediately, but does not reach a peak unless with prolonged and repeated use. This means that the immediate tolerance does not rise as high as with lysergamides or tryptamines and can wear off faster and can be reduced to half within 1-2 days in the absence of further consumption. Mostly there will be less psychedelic and more stimulating effects.
Extremely high doses of psychedelics can also produce a tolerance which can last a significantly longer time than expected.
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- Lithium - Lithium is commonly prescribed for the treatment of bipolar disorder. There is a large body of anecdotal evidence that suggests taking it with psychedelics significantly increases the risk of psychosis and seizures. As a result, this combination is strictly discouraged.
- Cannabis - Cannabis may have an unexpectedly strong and unpredictable synergy with the effects of Psychedelic. Caution is advised with this combination as it can significantly increase the risk of adverse psychological reactions like anxiety, paranoia, panic attacks, and psychosis. Users are advised to start off with only a fraction of their normal cannabis dose and take long breaks between hits to avoid unintentional overdose.
- Stimulants - Stimulants like amphetamine, cocaine or methylphenidate affect many parts of the brain and alter dopaminergic function. This combination can increase the risk of anxiety, paranoia, panic attacks, and thought loops. This interaction may also result in an elevated risk of mania and psychosis.[citation needed]
- Tramadol - Tramadol is well-documented to lower the seizure threshold[22] and psychedelics may act to trigger seizures in susceptible individuals.[citation needed]
See also
External links
Literature
- Nichols, D. E. (2016). Psychedelics. Pharmacological Reviews, 68(2), 264-355. https://doi.org/10.1124/pr.115.011478
- Geyer, M. A., Nichols, D. E., & Vollenweider, F. X. (2009). Serotonin-Related Psychedelic Drugs. Encyclopedia of Neuroscience. https://doi.org/10.1016/b978-008045046-9.01160-8
- Preller, K. H., & Vollenweider, F. X. (2016). Phenomenology, Structure, and Dynamic of Psychedelic States. https://doi.org/10.1007/7854_2016_459
- Vollenweider, F. X., & Kometer, M. (2010). The Neurobiology of Psychedelic Drugs: Implications for the Treatment of Mood Disorders. Nature Publishing Group, 11(9), 642–651. https://doi.org/10.1038/nrn2884
- Carhart-Harris, R. L., & Goodwin, G. M. (2017). The Therapeutic Potential of Psychedelic Drugs: Past, Present, and Future. Neuropsychopharmacology. https://doi.org/10.1038/npp.2017.84
- Nichols, C. D., & Sanders-Bush, E. (2001). Serotonin Receptor Signaling and Hallucinogenic Drug Action. Heffter Rev Psychedelic Res, 2, 73-79. https://web-beta.archive.org/web/20170110205041/https://heffter.org/docs/hrireview/02/chap5.pdf
- Johansen, P. Ø., & Krebs, T. S. (2015). Psychedelics not linked to mental health problems or suicidal behavior: A population study. Journal of Psychopharmacology, 29(3), 270-279. https://doi.org/10.1177/0269881114568039
- Elsey, J. W. (2017). Psychedelic drug use in healthy individuals: A review of benefits, costs, and implications for drug policy. Drug Science, Policy and Law, 3, 2050324517723232. https://doi.org/10.1177/2050324517723232
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Nichols, D. E. (April 2016). Barker, E. L., ed. "Psychedelics". Pharmacological Reviews. 68 (2): 264–355. doi:10.1124/pr.115.011478. ISSN 0031-6997.
- ↑ 2.0 2.1 Nichols, D. E., Nichols, C. D. (1 May 2008). "Serotonin Receptors". Chemical Reviews. 108 (5): 1614–1641. doi:10.1021/cr078224o. ISSN 0009-2665.
- ↑ Dictionary - Entheogen | http://dictionary.reference.com/browse/entheogen
- ↑ Strassmann, Rick (1984). "Adverse reactions to psychedelic drugs. A review of the literature". Journal of Nervous and Mental Disease. 172 (10): 577–595. doi:10.1097/00005053-198410000-00001. ISSN 0022-3018. OCLC 1754691. PMID 6384428.
- ↑ Murray, N. (2003). Aldous Huxley: a biography (1st U.S. ed ed.). Thomas Dunne Books/St. Martin’s Press. ISBN 9780312302375.
- ↑ "Humphry Osmond". BMJ. 328 (7441): 713. 20 March 2004. doi:10.1136/bmj.328.7441.713. ISSN 0959-8138.
- ↑ Weil, A., Rosen, W. (1993). From Chocolate to Morphine: Everything You Need to Know about Mind-altering Drugs. Houghton Mifflin. ISBN 9780395660799.
- ↑ Erowid Humphry Osmond Vault
- ↑ Martin, Douglas (2004-02-22). "Humphry Osmond, 86, Who Sought Medicinal Value in Psychedelic Drugs, Dies". New York Times. Retrieved 4 December 2010.
- ↑ Davis, W. (1996). One river: explorations and discoveries in the Amazon rain forest. Simon & Schuster. ISBN 9780684808864.
- ↑ Wasson, R. G., Hofmann, A., Ruck, C. A. P. (2008). The road to Eleusis: unveiling the secret of the mysteries (30th anniversary ed ed.). North Atlantic Books. ISBN 9781556437526.
- ↑ Petri, G., Expert, P., Turkheimer, F., Carhart-Harris, R., Nutt, D., Hellyer, P. J., Vaccarino, F. (6 December 2014). "Homological scaffolds of brain functional networks". Journal of The Royal Society Interface. 11 (101): 20140873. doi:10.1098/rsif.2014.0873. ISSN 1742-5689.
- ↑ Carhart-Harris, R. L., Muthukumaraswamy, S., Roseman, L., Kaelen, M., Droog, W., Murphy, K., Tagliazucchi, E., Schenberg, E. E., Nest, T., Orban, C., Leech, R., Williams, L. T., Williams, T. M., Bolstridge, M., Sessa, B., McGonigle, J., Sereno, M. I., Nichols, D., Hellyer, P. J., Hobden, P., Evans, J., Singh, K. D., Wise, R. G., Curran, H. V., Feilding, A., Nutt, D. J. (26 April 2016). "Neural correlates of the LSD experience revealed by multimodal neuroimaging". Proceedings of the National Academy of Sciences. 113 (17): 4853–4858. doi:10.1073/pnas.1518377113. ISSN 0027-8424.
- ↑ Vollenweider, F. X., Kometer, M. (September 2010). "The neurobiology of psychedelic drugs: implications for the treatment of mood disorders". Nature Reviews Neuroscience. 11 (9): 642–651. doi:10.1038/nrn2884. ISSN 1471-003X.
- ↑ Lawrence, J., Psychedelics: entering a new age of addiction therapy
- ↑ Nutt, D., King, L. A., Saulsbury, W., Blakemore, C. (March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". The Lancet. 369 (9566): 1047–1053. doi:10.1016/S0140-6736(07)60464-4. ISSN 0140-6736.
- ↑ 17.0 17.1 17.2 17.3 Nichols, D. E. (February 2004). "Hallucinogens". Pharmacology & Therapeutics. 101 (2): 131–181. doi:10.1016/j.pharmthera.2003.11.002. ISSN 0163-7258.
- ↑ Walterscheid, J. P., Phillips, G. T., Lopez, A. E., Gonsoulin, M. L., Chen, H.-H., Sanchez, L. A. (March 2014). "Pathological Findings in 2 Cases of Fatal 25I-NBOMe Toxicity". American Journal of Forensic Medicine & Pathology. 35 (1): 20–25. doi:10.1097/PAF.0000000000000082. ISSN 0195-7910.
- ↑ Kueppers, V. B., Cooke, C. T. (April 2015). "25I-NBOMe related death in Australia: A case report". Forensic Science International. 249: e15–e18. doi:10.1016/j.forsciint.2015.02.010. ISSN 0379-0738.
- ↑ Shanks, K. G., Sozio, T., Behonick, G. S. (October 2015). "Fatal Intoxications with 25B-NBOMe and 25I-NBOMe in Indiana During 2014". Journal of Analytical Toxicology. 39 (8): 602–606. doi:10.1093/jat/bkv058. ISSN 0146-4760.
- ↑ Diaz, J. (1997). How drugs influence behavior: a neuro behavioral approach. Prentice Hall. ISBN 9780023287640.
- ↑ Talaie, H.; Panahandeh, R.; Fayaznouri, M. R.; Asadi, Z.; Abdollahi, M. (2009). "Dose-independent occurrence of seizure with tramadol". Journal of Medical Toxicology. 5 (2): 63–67. doi:10.1007/BF03161089. ISSN 1556-9039.