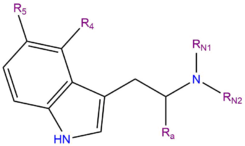
DMT
| Summary sheet: DMT |
| DMT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common names | DMT, N,N-DMT, Dmitry, The Glory, The Spirit Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substitutive name | N,N-Dimethyltryptamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Systematic name | 2-(1H-Indol-3-yl)-N,N-dimethylethanamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Psychoactive class | Psychedelic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical class | Tryptamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cannabis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stimulants | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tramadol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lithium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
A trip sitter is recommended with this substance that often is smoked in heavy doses. Short-acting intense trips may cause mental and physical side effects days after the hit has been taken. The overwhelming effects often require post-processing. It may take 2 days before physical side effects become noticeable, like shift in hearing. Using additional substances without a break (2 days + a few days to recover if you notice any side effects) increases the risk to get a bad post-trip. |
N,N-Dimethyltryptamine (also known as DMT, N,N-DMT, Dmitri, and "The Spirit Molecule") is a classical psychedelic substance of the tryptamine class.[1] Among psychedelics, it is known for its unique ability to produce short-lived but intense visionary states and complete hallucinations. It is thought to produce its effects by binding to serotonin receptors in the brain, although the precise mechanism is not fully understood.
DMT is present in over 65 species of plants and has been identified as being a normal constituent of human metabolism and an endogenous neurotransmitter in certain rodents. Its presence is also known to be widespread throughout the plant kingdom.[2][3] Although various theories have been postulated, its neurobiological function has yet to be determined.[citation needed]
When vaporized or smoked, DMT produces short-lived effects with a very rapid onset that is sometimes described as an "inconceivably high-speed rollercoaster ride." When ingested in combination with a MAOI or RIMA agent, it becomes active orally and significantly longer lasting, immersive, and interactive in nature: this combination is known as pharmahuasca. See also ayahuasca.
Unlike most highly prohibited substances, DMT has not been proven to be addictive or physiologically toxic.[1][4] However, adverse reactions such as severe anxiety, delusions and psychosis are always possible, even for experienced users, and particularly for those predisposed to mental disorders.[5]
It is highly advised to use harm reduction practices if using this substance.

History and culture
DMT was first synthesized in 1931 by the German chemist Richard Helmuth Fredrick Manske.[7][8] Its discovery as a natural product is generally credited to Brazilian chemist and microbiologist Oswaldo Gonçalves de Lima who, in 1946, isolated an alkaloid he named nigerina (nigerine) from the root bark of jurema preta (Mimosa tenuiflora).[8][9][10]
It was unequivocally identified in 1959, when American chemists were provided a sample of Mimosa tenuiflora.[10][11] In 1955, a team of American chemists led by Evan Horning isolated and formally identified DMT in the seeds and pods of Anadenanthera peregrina.[10][12]
Since 1955, the substance has been found in a host of organisms: in at least fifty plant species belonging to ten families,[2] and in at least four animal species, including one gorgonian[13] and three mammalian species.
Chemistry
DMT, or N,N-dimethyltryptamine, is a member of a family of organic compounds known as tryptamines. Tryptamines share a core structure consisting of a bicyclic indole heterocycle attached at R3 to an amino group via an ethyl side chain. DMT contains two methyl groups (CH3-) bound to the terminal amine RN at the end of this chain.
It has many homologs and analogs from base tryptamines like MET and DPT, to four and five position substituted variants such as 4-PO-DMT (psilocybin), 4-AcO-DMT (psilacetin), 5-HO-DMT (bufotenin), and 5-MeO-DMT.
Pure DMT is a white, crystalline solid that is often described as smelling like rubber. It is moderately soluble in water, but soluble in organic solvents and aqueous acids.[14]
Pharmacology
DMT's psychedelic effects are believed to come from its efficacy at the 5-HT2A receptor as a partial agonist. However, the role of these interactions and how they result in the psychedelic experience continues to remain elusive.
In addition to this, N,N-dimethyltryptamine is believed to be an endogenous ligand for the sigma receptor. However, the significance of the sigma-1 receptor remains the subject of ongoing scientific research.[15]
Limitations regarding MAO enzymes and MAO inhibitors
Unlike other tryptamines like psilocybin, orally administred DMT is inactive because the monoamine oxidase enzymes (MAO) break it down. To experience effects orally, DMT is frequently combined with MAO inhibitors (MAOIs), which prevent this breakdown. This combination, when done with both DMT and MAOI in their pure form, is known as pharmahuasca; when it's done through plant material, either brewed or ingested whole, it's traditionally referred as ayahuasca[16].
While insufflated DMT has a fast enough onset and come up to provide a solid experience similarly to smoked DMT, doing so could stimulate the production of liquid secretions that might get mixed with it, which can drip down the throat and be swallowed, meaning that it will be broken down in the stomach and wont contribute to the experience.
The consumption of ayahuasca implies that the DMT and the harmala alkaloids are ingested simultaneously. Despite this being the traditional way to do ayahuasca, it's not the most efficient way when it comes to maximize the absorption of the psychedelic drug. Both DMT and harmala alkaloids have very similar pharmacokinetics[17], but modern users tend to use lower doses of harmala alkaloids and higher doses of DMT when combining both drugs; this way, DMT tends to reach the peak sooner than the MAOI used. In such occasions, a way to optimize the employment of MAO inhibitors is to ingest them about 30 minutes before the consumption of the DMT. The time window for the consumption of the MAOI is 10 to 50 minutes before the ingestion of the psychedelic drug.
Variety of DMT methods and routes of administration
DMT is a drug that presents one of the most wide and varied methodology when it comes to methods and routes of administration. A DMT experience can be anything from an aggressive and brief yet trascendental trip of intravenous DMT to a extensive, smooth and interactive ayahuasca experience. It's better to classify these kinds of different experience in terms of their duration into short and long-acting DMT trips (from now referred as "short DMT trips" and "long DMT trips" respectively), being the long DMT trips those that can be induced through inhibition of MAO enzymes. The followings are all the different ways in which DMT can be consumed: [18][19][20]
- Short-acting DMT trip consumptions:
- Smoked/vaporized DMT (from 10 to 60 mg; from 5 to 20 minutes)
- Intravenous injection of DMT (from 4 to 20 mg; from 10 to 30 minutes)
- Smoked changa (dosage difficult to measure, recommended to follow smoked DMT dosage range; up to 35 minutes)
- Intramuscular injection of DMT (up to 80 mg; up to 45 minutes)
- Intranasal administration of DMT (unknown dosage ranges, presumed similar to those of smoked/vaporized DMT, unknown duration)
- Ayahuasca and equivalent preparations consumptions:
- Oral ingestion of MHRB (mimosa hostilis root bark) brew or plant material (up to 50 g of ~1.2% MHRB; up to 2.5 hours)[21]
- Oral ingestion of ayahuasca or equivalent preparations, either liquid brew or solid plant material:
- Harmala alkaloids-containing plant, frequently baniseriopsis caapi or peganum harmala (amount of plant material with an equivalence of 140 to 240 mg of total alkaloids; up to 50 minutes prior to the ingestion of the DMT-containing brew or plant material, or simultaneously with it)
- DMT-containing plant, frequently mimosa hostilis, acacia confusa or psychotria viridis (amount of plant material with an equivalence of 35 to 85 mg; from 5 to 10 hours)
- Oral ingestion of pharmahuasca:
- MAOI or RIMA (from 140 to 240 mg of total harmala alkaloids, or an equivalent amount of harmalas-containing plant, or an equivalent dose of a pharmaceutical MAOI or RIMA; up to 50 minutes prior to the ingestion of DMT or simultaneously with it)
- DMT (from 35 to 85 mg; from 5 to 10 hours)
- Long-acting DMT trip consumptions (for each: a co-administration through the same ROA of 70 to 150 mg of total harmala alkaloids[22], or an oral ingestion of 140 to 240 mg of total alkaloids, or an equivalent amount of harmalas-containing plant material, or an equivalent dose of a pharmaceutical MAOI or RIMA; from 70 to 30 minutes prior to the administration of DMT crystals):
- Rectal administration of DMT crystals (up to 70 mg; from 5 to 8 hours)[23]
- Sublingual administration of DMT crystal (unknown dosage ranges, pressumed similar to those of rectal DMT; unknown duration)
- Intranasal administration of DMT crystal (unkown dosage ranges, pressumed similar to those of smoked/vaporized DMT; unknown duration)
- Hypothetical "long DMT trip" consumptions (for each: an oral ingestion of either 140 to 240 mg of total harmala alkaloids, or an equivalent amount of harmalas-containing plant material, or an equivalent dose of a pharmaceutical MAOI or RIMA):
- Smoked/vaporized DMT (from 10 to 60 mg; unknown duration, pressumed higher than that of changa)
- Intravenous injection of DMT (from 4 to 20 mg; unknown duration, assumed higher than that of intravenous DMT without prior harmalas ingestion)
- Intramuscular injection of DMT (up to 80 mg; unknown duration, assumed higher than that of intramuscular DMT without prior harmalas ingestion)
Subjective effects
Depending on the dosage and method of administration, the effects of DMT can range from mild psychedelic states to powerfully immersive life-altering experiences which are often described as the ultimate displacement from ordinary consciousness in which users report experiencing ineffable spiritual realms or alternate dimensions. It's also commonly reported to encounter 'beings' of unknown origin after consuming a high dose of DMT. Terrence Mckenna and Dr. Rick Strassman have both studied and popularized this phenomenon. [24]
DMT in its smokeable form is reported to be the least mentally inebriating psychedelic. It is due to a lack of perceived intoxication that many people describe DMT as a genuine experience that is actually happening to them.
It is worth noting that many people report that smoked DMT is extremely clear-headed in its style and tends to produce less personal insight in comparison to orally active psychedelics such as ayahuasca, LSD and psilocybin due to its short-acting nature.
An important detail that must be considered is that the majority of the following information about the effects that DMT can produce is extracted almost entirely from reports of smoked DMT. It's completely possible to induce a DMT trip with the co-ingestion of a MAOI but employing a different ROA than oral (the latter referred as ayahuasca or pharmahuasca), for example: rectal, sublingual or intranasal. Such methods of consumption will allow the DMT to avoid passing through the liver and also inhibit the action of the MAO enzymes, leading to "long DMT trips" that can provide a series of effects that are distinct to those of smoked DMT but that also differ slightly from classical ayahuasca experiences.
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Changes in felt bodily form[9]
- Increased heart rate[9]
- Increased blood pressure[25]
- Neurogenesis[26] and Neuroplasticity[27] - Recent studies have shown that psychedelics, when done in a therapeutic setting and following harm reduction practices, can improve brain health by promoting neuron production and increasing plasticity.
- Pupil dilation[9]
- Physical autonomy
- Spatial disorientation[9]
- Temperature regulation suppression
- Spontaneous bodily sensations - The "body high" of DMT can be described as a pleasurable all-encompassing glow. It maintains a consistent presence that quickly rises with the onset and hits its limit once the peak has been reached. It is capable of becoming very powerful at higher doses and can remain after the experience itself has ended for up the same amount of time of the trip's entire duration.
- Physical euphoria - It should be noted that, when smoking DMT, this effect is not as reliably produced as it is with substances like stimulants or entactogens, and can just as easily manifest as physical discomfort for no apparent reason. However, even if such discomfort manifests during a "long DMT trip", it tends to eventually fade into a distinctive physical euphoria when the come down is approaching. Such euphoria is described as an all encompasing inner warmth which can, ocasionally, become very powerful, in a fashion that would be described as "more blissful than opioids like heroin or oxycodone".
- Changes in felt gravity - When smoking higher DMT breakthrough doses, physical feelings of being launched across vast distances at incredibly high speeds are commonly reported.[9]
- Nausea - This effect is much less common than it is with 5-MeO-DMT as well as longer-lasting psychedelics like psilocybin mushrooms or mescaline. However, it can still manifest spontaneously and sometimes lead to sudden bouts of vomiting, particularly when a MAO inhibitor has been used to induce a "long DMT trip". Fasting during the previous hours to the experience or eating small portions (and preferently light foods) can prevent this effect.
- Increased libido
- Seizure - This is a very rare effect but is believed to happen in those who are predisposed to them, especially while in physically taxing conditions such as being dehydrated, fatigued or undernourished.
Visual effects 
-
It has been proposed that DMT visual effects will increasingly show up during the come up of the experience but will reach a peak of hallucinations that can last about ten to twenty minutes after the experience started, and after that, despite the cognitive effects will still be in the peak, the visual effects seem to start fading slowly during the rest of the experience.
Since smoked DMT provides an experience that doesn't last more than half an hour, the visual effects disappear at the same time than the rest of the effects, but with rectally, sublingually or intranasally active DMT, the user can notice the slow decrease of visual effects even if they're experiencing an ego death.
Enhancements
Distortions
- Drifting (melting, breathing, morphing and flowing) - In comparison to other psychedelics, this effect can be described as highly detailed, slow and smooth in motion and static in its appearance.
- Colour replacement
- Colour shifting
- Colour tinting
- Tracers and after images - For most users, these are probably the most long-lasting visual effects of DMT, and can still be noticed even when towards the tail end of the entire experience.
- Recursion
- Scenery slicing
- Symmetrical texture repetition
- Environmental patterning
Geometry
The visual geometry encountered can be described as more similar in appearance to that of psilocin than LSD. It can be comprehensively described through its variations as intricate in complexity, abstract in form, equally organic and digital in feel, structured in organization, brightly lit, multicoloured in scheme, glossy in shading, equal in sharp and soft edges, large in size, fast in speed, smooth in motion, equal in rounded and angular corners, immersive in depth and consistent in its intensity. At higher doses, it is significantly more likely to result in states of level 8B visual geometry over level 8A.
The geometry present with smokeable DMT is considered by many to be the most profoundly intricate and complex set of visual geometry found within the entirety of the psychedelic experience. In comparison to ayahuasca (or other forms of active DMT), it is significantly more digital in appearance and contains a colour scheme which is similar to LSD and a structured style that resembles a high dose of psilocin (4-HO-DMT).
Hallucinatory states
DMT produces a full range of high level hallucinatory states in a fashion that is more consistent and reproducible than that of any other commonly used psychedelic. These effects include:
- Machinescapes
- Transformations
- Internal hallucination (autonomous entities; settings, sceneries, and landscapes; perspective hallucinations and scenarios and plots) - DMT produces high level internal hallucinations at appropriate doses more consistently than that of any other psychedelic. They are more common within dark environments and can be comprehensively described through their variations as lucid in believability, interactive in style, new experiences in content, autonomous in controllability, geometry-based in style and almost exclusively of a personal, religious, spiritual, science-fiction, fantasy, surreal, nonsensical or transcendental nature in their overall theme.
- External hallucination (autonomous entities; settings, sceneries, and landscapes; perspective hallucinations and scenarios and plots) - These are more common within dark environments and can be comprehensively described through their variations as lucid in believability, interactive in style, new experiences in content, autonomous in controllability, geometry-based in style and almost exclusively of a personal, religious, spiritual, science-fiction, fantasy, surreal, nonsensical or transcendental nature in their overall theme.
Auditory effects 
-
- Enhancements - Enhancements of one's auditory acuity, often following the end of the experience, have been reported in clinical studies with intravenously-administered DMT.[9]
- Distortions[9]
- Hallucinations
Multi-sensory effects 
-
- Synaesthesia - In its fullest manifestation, this is a very rare and non-reproducible effect. Increasing the dosage can increase the likelihood of this occurring, but seems to only be a prominent part of the experience among those who are already predisposed to synaesthetic states.
Cognitive effects 
-
- Anxiety - Anxiety seems to manifest more consistently with DMT in comparison to other psychedelics like LSD or psilocybin and it's oftenly present during the come up phase, fading off while the user enters the peak. This effect may be related to the drug's capacity to produce high levels of memory suppression given that the feeling itself is oftenly described as if one's consciousness is losing touch with reality or that the mind is being ripped apart. In very high doses the user may suffer the percepton that is being driven to insanity against their will, getting disintegrated or even fall into the not uncommon delusion that is dying. The practice of grounding exercises can help the user to deal with this effect.
- Feelings of impending doom[9]
- Pre-trip anxiety - While this effect can be manifested during the experience itself -particularly during the come up-, it's extremely common for nervousness, anxiety or fear to be suffered minutes, hours or sometimes even days prior to the experience. This effect is described as if the user is about to face, alone, an inminent danger, and depending on the set and setting, the level of experience and how often the user "trips", this effect can vary from mild to overwhelming, which can lead to the user's decision to delay or decline the consumption of the drug. It's discouraged to users to make use of anxyolitic drugs to inhibe this effect since the experience can be significantly "numbed", making the user to regret such decision.
- Déjà vu
- Delusion - Delusions can be presented alongside personal meaning intensification, and are common among inexperienced users, who may be not completely aware that this is an effect on itself and not necessarily a strict implication that feeling a connection or implication of any kind means that there is actually one.
- Ego replacement
- Emotion enhancement - While smoking DMT doesn't present this effect with enough consistency, the co-consumption of a MAOI will lead to a prolonged "trip" in which the user can experience common emotions in an intensified fashion, and even if so called negative emotions such as sadness or melancholy can appear during the experience -depending on the set, setting and background context-, it's common for the experience to still be defined as "pleasant" since such emotions often produce catharsis and can be properly dealt through personal bias suppression.
- Empathy, affection, and sociability enhancement - On "short DMT trips", this effect differs from MDMA and other entactogens in that it isn't as central to the experience and it's mostly presented as a part of the afterglow. It can manifest more consistently during "long DMT trips", where it feels more natural and less forced, and is experienced predominantly during the come down or after the experience ended.
- Euthymia - This effect manifests itself acutely for all classical psychedelics when one to three doses are combined with a psychotherapy treatment program. In case of DMT, euthymia has a slightly higher ocurrence when a MAOI is added to the ingestion. When comparing meta analyses, psychedelic psychotherapy greatly outperforms "gold standard" treatments for several mental health problems.
- Catharsis - While smoking DMT doesn't provide a long enough experience to process trauma or difficult situations while under the influence of the drug, the co-consumption of a MAOI alongside the non-oral DMT will extend the experience duration and some users that have been going through certain traumatic backgrounds can experience a process of "healing" and "letting go" in a very similar manner to ayahuasca.
- Analysis, creativity and immersion enhancements - While smoking DMT can produce these enhancements, these are not as noticeable as with "long DMT trips", given that such experiences will last long enough for the user to get involved in activities where the effects can be much more appreciated. In these situations, these effects are similar to those of LSD, psilocybin or ayahuasca.
- Cognitive euphoria - When applied a "long trip" method, DMT can induce this effect with one of the highest consistencies among psychedelics, being sometimes described as "pure bliss or ecstasy". It's commonly reported that, when the peak starts transitioning into the come down of the experience, feelings of peace, fulfillment and appreciation will become central to the experience. Sometimes and depending on the set and setting, this effect can be accompanied by an existential self-realization and/or feelings of unity and interconnectedness, which occasionally can lead to the user to a trascendental moment, even if the peak itself of the whole experience has already passed.
- Increased music appreciation - When DMT is smoked or vaporized, this effect typically occurs only at sub-breakthrough doses, and many people prefer to have their DMT experiences in complete silence to prevent a muddled or overwhelming experience. On the other side, when a MAOI is added and DMT is administred rectally, sublingually or intranasally, this effect can become as prominent as with LSD or psilocybin and sometimes will be central to the experience, especially during the come down.
- Memory formation enhancement - While many users experience amnesia during the peak of the experience, one can recall certain events of the trip with an increased facility, in comparison to the great majority of other drugs or everyday life. This effect can persist during the following hours or days after the experience.
- Memory suppression
- Mindfulness - Mindfulness tends to occur after the experience has ended and the individual has returned to ordinary waking consciousness, to a sense of presence and sensitivity towards one's inner sensations as well as outer environment. This effect can last significantly longer once the experience has ended if the user went through a "long DMT trip" by using a MAOI to extend the experience's duration.
- Multiple thought streams - This effect tends to manifest in a much more chaotic fashion, in tandem with the sensation of cognitive overload.
- Novelty enhancement - Similarly to cognitive euphoria, novelty enhancement is another signature effect of a "long DMT trip". The user might adopt a "childish enthusiasm" towards their environment, becoming sensitive to elements that in other conditions would be defined as trivial, common or even boring, such as the blue color of the sky, the texture of a blanket or a joke told by someone else.
- Personal bias suppression
- Suggestibility enhancement
- Rejuvenation - This effect tends to occur after the experience has ended and the subject has returned to ordinary waking consciousness, often in Near-Death Experience (NDE) variants of a DMT experience.
- Appetite suppression and decreased libido - Despite the fact that both effects are commonly referred as physical effects, "long DMT trips" are capable to leave the user in a status of overall satisfaction and contentment, thus decreasing any urge to eat or take part in sexual activities, even if the user is perfectly capable of doing so in physical terms. This effect, alongside mindfulness is one of the common effects of the afterglow and it has been reported to last for several days or weeks even.
- Autonomous voice communication
- Conceptual thinking - During "breakthrough" doses, this effect is prominent due to the intense memory suppression that can manifest, leading users to have "thoughts that cannot be spoken".
- Thought acceleration, connectivity and organization
- Time distortion - When smoking, this particular effect is most prevalent and notable with "breakthrough" experiences.[9], and only tends to last under fifteen minutes but is commonly reported to subjectively feel as if it had lasted much longer, in some cases "many lifetimes" or even an "eternity". On the other hand, during "long DMT trips", while the user can still experience an "eternal" breakthrough experience, after the peak ends and the user enters the comedown, they can still feel that time is passing slower than usual; this effect is related to other effects like analysis enhancement and immersion enhancement.
- Wakefulness - The administration of DMT with a MAOI can extend the effects for long enough to suppress the user's urge to sleep.
Transpersonal effects 
-
For a number of individuals these effects are consistently more reproducible and powerful with DMT than they are with other “classical psychedelics” such as LSD or mescaline, this is most likely due to its very intense effects. These components are unique to DMT in that for a majority of its users they are significantly more likely to manifest during "breakthrough" experiences as opposed to sub-breakthrough level experiences.
- Spirituality enhancement
- Existential self-realization - This effect makes the everyday instant assumptions such as being alive or having loved ones around to be reprocessed, making the user more aware of their own situation and leading to feelings of gratitude.
- Near-death experience[28][29]
- Perception of eternalism
- Perception of self-design
- Perceived exposure to inner mechanics of consciousness
- Unity and interconnectedness
Progressive stages of a short-acting DMT experience
When smoked or vaporized at moderate to heavy dosages, the DMT experience consistently manifests itself in a progressive sequence which can be described as follows:
1. "Breaking Through"
The first step of a DMT trip is the come up that leads onto an experience commonly referred to as "breaking through." This seems to have at least a few different ways of presenting itself to the user.
The first thing that a person notices is an extremely distinct set of visual enhancements such an increase in visual acuity and colour intensity. This is followed by a sudden onset of high level 3 geometry which increases in its intensity until it envelopes and covers the external environment. These effects are often accompanied by auditory hallucinations such as soft crackling sounds or high pitched extended tones. There is also the possibility of accompanying physical sensations as one "breaks through." These can include feelings of suddenly being pushed through and onto the other side of a membrane or feelings of shooting through space at high speeds.
2. "The Waiting Room"
Almost immediately after a person has inhaled enough DMT to have "broken through", they often find themselves spending a brief amount of time in what is sometimes described as a psychedelic "waiting room" or "loading screen". The appearance of this space can assume a seemingly infinite variety of forms but generally appears in the shape of an enveloping tunnel comprised of rapidly shifting, interlocking geometry. This lasts approximately 10 to 20 seconds and feels qualitatively different from other stages of the experience.
3. "The Other Side"
Once the waiting period is over, the user will feel that they have "broken through" onto the other side. It is here where users experience intense level 7 geometry and level 3 - 4 internal hallucinations. It is worth noting that although experiences vary between individuals, DMT trips often follow common archetypes, scenarios, content, and plots. These scenarios generally consist of visiting what appears to be an alternate reality that is often described to contain autonomous entities, settings, sceneries, and landscapes as well as themes of a cosmic, transcendental, or transpersonal nature.
4. "Drifting Down"
The final stage is experienced as the sensation of being pulled further and further away from the scenario until it is no longer visible and one finds themselves back in reality. This is typically accompanied by level 3 - 4 geometry as well as a sense of general exhilaration and awe. The moderate to mild geometry stays for a further 10 – 15 minutes before disappearing completely, sometimes leaving a noticeable "body high" that lingers for up to an hour.
Progressive stages of a long-acting DMT experience
"Long DMT trips" are reported to be at least twice as long as "short DMT trips" and have particular effects that oftenly don't take place when the drug is smoked in the absence of a MAOI. A "long DMT trip" can be described as a four phase experience, with a come up, a peak divided in two parts and a come down:
1. Come up
Depending on the route of administration, the come up phase can last about 5 to 20 minutes. Since for this methodology the DMT is commonly prepared in the form of acetate (which can extend the onset duration to several minutes) and administrated rectally, nasally or sublingually, the user is not "busy" trying to vaporize crystals or manipulating a syringe while the effects are "wearing on", meaning that they can be in a state of relaxation through all the come up. Given that this phase is longer than during a "short DMT trip", it doesn't present the same distress and can be dealt with proper calm and better focus. It can still, though, provoke anxiety and give the classical delusion of dying. The first effect that is usually noticed -and that "marks" the beginning of the experience- is a tingling sensation which is perceived as located in the brain. Other common effects are multiple thought streams and memory suppression.
2. "The longer breakthrough"
The rectal, sublingual and intranasal routes of administration are all capable of producing breakthroughs, which can be very intense and are usually at least 30 minutes long and can last up to 2 hours, depending on the dosages (quantity and presentation). A route of administration such as intranasal has a fast onset and come up and, during the first 15 minutes of the peak, can provide a very similar experience than smoked DMT if a high dose is used. Slower ROAs, such as rectally or sublingually, tend to give so called "ayahuasca breakthroughs" but usually shorter and with less body load, as well as a higher intensity and clean headspace. Nonetheless, given that all "long DMT trip" methodologies are proficient in inducing "longer breakthroughs", it can become draining for the user to be in an extended period of time in such an altered state of consciousness; users prefer to use sub-breakthrough doses to have experiences of still a high intensity but in less degree than that of a typical "breakthrough". The "long DMT trip breakthrough" can present both smoked DMT and ayahuasca typical geometry, alongside conceptual thinking, time distortion, and sometimes unity and interconnectedness, ego death or a near-death experience.
3. "Coming back"
If the user has experienced a breakthrough, a second part in the peak can be present as a short period of time (usually lasting from 20 to 60 minutes) in which the user is still experiencing some "breakthrough" effects but also being in a roughly "normal awareness". Common tasks are difficult to perform since the effects taking place are still too distracting and the user feels sedated, but walking around and talking with people is already doable without experiencing a noticeable amnesia. This part is more of a transition between the "breakthrough" and the come down.
4. Come down
Despite DMT is known due to the reports of trascendental breakthroughs, users that try the "long DMT trip" method frequently find the come down phase to be the most pleasant part of the experience. It's also the longest part of the experience, usually lasting up to 4 hours. This type of DMT experience is unique due to the fact that people are totally still under the effects of DMT but without going through a breakthrough, meaning that now they can interact with the world and get involved in activites in a very particular state of mind, frequently stronger than that of psilocybin. The most prominent effects of this phase are cognitive euphoria, social enhancement and increased music appreciation. Other common effects are rejuvenation and novelty enhancement, and also analysis, creativity and immersion enhancements.
Experience reports
Anecdotal reports which describe the effects of this compound within our experience index include:
- Experience: 100ug ALD-52 - Nice weekend trip
- Experience: 25mg 50% DMT Changa + Cannabis (Smoked - Bong) - Insights into my consciousness
- Experience:2.5g Mushrooms + 500mg DMT
- Experience:25mg DMT - Your wall can't save you
- Experience:300mg DXM + 25mg DMT + Cannabis - A crazy night
- Experience:30mg (smoked) DMT - The Monolith
- Experience:337mg DMT fumarate - A Day With DMT
- Experience:40mg (smoked) - The planet became a watermelon
- Experience:40mg DMT - Second breakthrough
- Experience:40mg smoked - Ego death and unity with a friend
- Experience:50mg - Demon faces and déjà vu
- Experience:50mg - Truth
- Experience:75mg DMT - Experiencing Death
- Experience:80mg - progressing deeper into states of unity and interconnectedness
- Experience:An Excessive Amount - N,N DMT / Marijuana - Stuck Inside an Egg
- Experience:DMT (30mg, glass pipe) - First DMT trip ever turned therapeutic
- Experience:DMT (60mg, freebase) - passing out
- Experience:DMT (75 mg, smoked) - Bad trip with illustration
- Experience:DMT (unknown dose, smoked) - It felt like I was on rails the whole time
- Experience:DMT (unknown, smoked/vaporized) - She Struggles then Finds Beauty and the Nature of DMT Itself
- Experience:DMT (unknown, smoked/vaporized) - Unexpected Correspondences
- Experience:DMT (~50mg) + Cannabis - Geometric Angels
- Experience:DMT: 1,1mg 1/5 changa x3 – The continuation
- Experience:DMT: 200mg 1/5 changa - Bad yet glorious trip
- Experience:DXM (340 mg) + DMT (30 mg, smoked) + Cannabis - Amazing Synergy
- Experience:MDMA (750mg, Oral) - Finally Free
Additional experience reports can be found here:
Natural plant sources
Mimosa hostilis root bark
Mimosa hostilis (also known as Mimosa tenuiflora, Jurema and Tepezcohuite) is a perennial tree or shrub native to the northeastern region of Brazil and is found as far north as southern Mexico. Around 1% of the dried weight is DMT. It is legal to purchase online in many parts of the world and a commonly used source for performing DMT extractions or brewing into ayahuasca.
Acacia confusa root bark
Acacia confusa (also known as Acacia Petit Feuille, Small Philippine Acacia, Formosa Acacia (Taiwan Acacia) and Formosan Koa) is a perennial tree native to South-East Asia. It is legal to purchase online and easily accessible in many parts of the world. The plant matter itself contains the following chemicals:[citation needed]
- N-Methyltryptamine: 1.43% (not psychoactive without MAOI)
- DMT: 1.15%
Preparation methods
Preparation methods for this substance found within our tutorial index include:
- DMT extraction using sodium hydroxide and naphtha
- Acid-base DMT extraction, based on Marsofold's tek
- D-Limonene and vinegar DMT extraction
- Norman Tek
- DMT ingestion methods
Research
Dr. Rick Strassman has hypothesized that the pineal gland is responsible for the production and release of DMT which he believes possibly could be excreted in large quantities at the moments of birth and death.[30] However, this view was contested by David E. Nichols in 2018, who argued that the pineal gland secretes insufficient amounts of DMT to produce psychoactive effects.[31]
In 2019, a study by Jimo Borjigin demonstrated in rat brains that brain neurons with the two enzymes required to make DMT were not just in the pineal gland but also in the neocortex and hippocampus.[32]
Near-death experience
A 2018 study found significant relationships between a DMT experience and a near-death experience.[28] A 2019 large-scale study found that ketamine, Salvia divinorum, and DMT (and other classical psychedelic substances) are linked to near-death experiences.[29]
Neurogenesis
In September 2020, an in vitro and in vivo study showed that DMT present in the ayahuasca infusion promotes neurogenesis.[26]
Neuroplasticity
In 2018, a study demonstrated neuroplasticity induced by DMT and other psychedelics through TrkB, mTOR, and 5-HT2A signaling.[33]
Reagent results
Exposing compounds to the reagents gives a colour change which is indicative of the compound under test.
| Marquis | Mecke | Mandelin | Liebermann | Froehde | Robadope | Ehrlich | Hofmann | Simon's |
|---|---|---|---|---|---|---|---|---|
| Yellow - Orange - Brown | Yellow - Green - Dark green | (faint) Green - Brown | Black | No reaction | No reaction | Pink - magenta | Yellow | No reaction |
Toxicity and harm potential
 |
This toxicity and harm potential section is a stub. As a result, it may contain incomplete or even dangerously wrong information! You can help by expanding upon or correcting it. |
DMT is considered to be non-addictive, is not associated with any form of neurotoxicity, and has an extremely low toxicity relative to dose. As with other psychedelic substances, there are relatively few physical side effects associated with acute DMT exposure. Various studies have shown that in reasonable doses in a careful context, it has little to no negative cognitive, psychiatric or physical consequences.[9]
However, as with psychedelics generally, DMT is thought to be able to act as a potential trigger for those with underlying psychiatric conditions. Those with a personal or family history of mental illness are advised not to use this substance unless under medical supervision.
Despite the lack of physical risks, it is highly advised to approach this substance with extreme caution and harm reduction practices.
Lethal dosage
The median lethal dose (LD50) of DMT in humans has never been reached in any setting, nor is this expected to change due to its pharmacological properties.
Dependence and abuse potential
Like other serotonergic psychedelics, DMT is considered to be non-addictive with a low abuse potential.[9] There are no literature reports of successful attempts to train animals to self-administer DMT — an animal model predictive of abuse liability — indicating that it does not have the necessary pharmacology to either initiate or maintain dependence. Likewise, there is virtually no withdrawal syndrome when chronic use of DMT is stopped.[citation needed]
Notably, tolerance to the effects of DMT does not appear to occur. The reason for this is unknown. Likewise, DMT does not produce cross-tolerance with other psychedelics, meaning that after the consumption of DMT, psychedelics will not have a reduced effect.
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- Lithium - Lithium is commonly prescribed for the treatment of bipolar disorder. There is a large body of anecdotal evidence that suggests taking it with psychedelics significantly increases the risk of psychosis and seizures. As a result, this combination is strictly discouraged.
- Cannabis - Cannabis may have an unexpectedly strong and unpredictable synergy with the effects of DMT. Caution is advised with this combination as it can significantly increase the risk of adverse psychological reactions like anxiety, paranoia, panic attacks, and psychosis. Users are advised to start off with only a fraction of their normal cannabis dose and take long breaks between hits to avoid unintentional overdose.
- Stimulants - Stimulants like amphetamine, cocaine or methylphenidate affect many parts of the brain and alter dopaminergic function. This combination can increase the risk of anxiety, paranoia, panic attacks, and thought loops. This interaction may also result in an elevated risk of mania and psychosis.[citation needed]
- Tramadol - Tramadol is well-documented to lower the seizure threshold[34] and psychedelics may act to trigger seizures in susceptible individuals.[citation needed]
Legal status
Internationally, DMT is classified as a Schedule I controlled substance under the United Nations 1971 Convention on Psychotropic Substances, meaning that international trade in DMT is supposed to be closely monitored and its use is supposed to be restricted to scientific research and medical use. Natural materials containing DMT, including ayahuasca, are not regulated under the 1971 Psychotropic Convention.[35][36]
- Australia: DMT is controlled under Schedule 9 of the Poisions Standard.[37] A schedule 9 drug is outlined in the Poisons Act 1964 as "Substances which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of the CEO."[38] Under the Misuse of Drugs act 1981 6.0 g of DMT is considered enough to determine a court of trial and 2.0 g is considered intent to sell and supply.[39] Between 2011 and 2012, the Australian Federal Government was considering changes to the Australian Criminal Code that would classify any plants containing any amount of DMT as "controlled plants".[40] DMT itself was already controlled under current laws. The proposed changes included other similar blanket bans for other substances, such as a ban on any and all plants containing Mescaline or Ephedrine. The proposal was not pursued after political embarrassment on realisation that this would make the official Floral Emblem of Australia, Acacia pycnantha (Golden Wattle), illegal. The Therapeutic Goods Administration and federal authority had considered a motion to ban the same, but this was withdrawn in May 2012 (as DMT may still hold potential entheogenic value to native and/or religious people).[41]
- Austria: DMT is illegal to possess, produce and sell under the SMG (Suchtmittelgesetz Österreich).[citation needed]
- Belgium: DMT is illegal to possess, sell, purchase and import.[42]
- Brazil: DMT is illegal to possess, produce and sell under Portaria SVS/MS nº 344.[43] Rules are relaxed regarding religious use.[citation needed]
- Canada: DMT is listed in Schedule III of the Controlled Drugs and Substances Act.[44]
- Denmark: DMT is a Category B controlled substance.[45]
- Estonia: DMT is a Schedule I controlled substance.[citation needed]
- France: DMT is classified as a narcotic.[citation needed]
- Hungary: DMT is illegal to possess, produce and sell.[citation needed]
- Germany: DMT is controlled under Anlage I BtMG (Narcotics Act, Schedule I)[46] as of January 24, 1974.[47] It is illegal to manufacture, possess, import, export, buy, sell, procure or dispense it without a license.[48]
- Ireland: DMT is a Schedule 1 controlled substance under the Misuse of Drugs Acts.[49]
- Israel: DMT is an illegal substance. Production, trade and possession are prosecuted as crimes.[50]
- India: DMT is illegal to produce, transport, trade in or possess.[51]
- Italy: DMT is a Schedule I controlled substance.[52]
- Latvia: DMT is a Schedule I controlled substance.[53]
- The Netherlands: DMT is classified as a Lijst 1 (List I) controlled substance under the Opiumwet (Opium Law).[54]
- New Zealand: DMT is classified in New Zealand as a Class A controlled substance under the Misuse of Drugs Act 1975.[55]
- Norway: DMT is a Schedule I controlled substance.[citation needed]
- Russia: DMT is a список I (List I) contolled substance. It is illegal to possess, produce and sell.[56]
- Serbia: DMT is a List 4 controlled substance.[citation needed]
- Sweden: DMT is controlled under Förteckning I (Schedule 1).[57] The Swedish supreme court concluded in 2018 that possession of processed plant material containing a significant amount of DMT is illegal. However, possession of unprocessed such plant material was ruled legal.[citation needed]
- Switzerland: DMT is a controlled substance specifically named under Verzeichnis D.[58]
- United Kingdom: DMT is a Class A controlled substance.[59]
- United States: DMT is a Schedule I controlled substance.[60] Rules are relaxed regarding religious use, however. In the US, dried root bark of Mimosa hostilis had been considered a "grey area" item for a long time. However, recent efforts by the DEA appear to be focusing on eliminating internet sales of the bark, citing 21 USC § 841, which states that "(IV) any compound, mixture, or preparation which contains any quantity of any of the substances referred to in subclauses (I) through (III)" is also considered an illegal substance. Many USA based vendors have since been stocking Acacia confusa bark as a result due to its very similar alkaloid content.[citation needed]
- Czech Republic: DMT is a Schedule I controlled substance.[61]
See also
External links
Discussion
Literature
- Nichols, D. E. (2016). Psychedelics, (April), 264–355. https://doi.org/10.1124/pr.115.011478
- Strassman, R. J. (1995). Human psychopharmacology of N, N-dimethyltryptamine. Behavioural Brain Research, 73(1), 121-124. https://doi.org/10.1016/0166-4328(96)00081-2
- Strassman, R. J., Qualls, C. R., Uhlenhuth, E. H., & Kellner, R. (1994). Dose-Response Study of N,N-Dimethyltryptamine in Humans: II. Subjective Effects and Preliminary Results of a New Rating Scale. Archives of General Psychiatry, 51(2), 98-108. PMID: 8297217.
References
- ↑ 1.0 1.1 Nichols, David E. (2016). Barker, Eric L., ed. "Psychedelics". Pharmacological Reviews. 68 (2): 264–355. doi:10.1124/pr.115.011478. eISSN 1521-0081. ISSN 0031-6997.
- ↑ 2.0 2.1 Ott, Jonathan (1994). Ayahuasca Analogues: Pangæan Entheogens (1st ed.). Kennewick, WA, USA: Natural Products. pp. 81–83. ISBN 978-0-9614234-5-2. OCLC 32895480.
- ↑ Shulgin, Alexander; Shulgin, Ann (1997). "DMT is Everywhere". TiHKAL: The Continuation. United States: Transform Press. p. 277. ISBN 0-9630096-9-9. OCLC 38503252.
- ↑ Lüscher, Christian; Ungless, Mark A. (2006). "The Mechanistic Classification of Addictive Drugs". PLOS Medicine. 3 (11). doi:10.1371/journal.pmed.0030437. ISSN 1549-1277. PMID 17105338.
- ↑ Strassmann, Rick (1984). "Adverse reactions to psychedelic drugs. A review of the literature". Journal of Nervous and Mental Disease. 172 (10): 577–595. doi:10.1097/00005053-198410000-00001. ISSN 0022-3018. OCLC 1754691. PMID 6384428.
- ↑ "q21q21" (September 19, 2014). "Q21Q21 tek (and other limeteks) NOT recommended for shredded bark!". DMT Nexus. Retrieved January 8, 2020.
- ↑ Manske R. H. F. (1931). "A synthesis of the methyltryptamines and some derivatives". Canadian Journal of Research. 5 (5): 592–600. doi:10.1139/cjr31-097. ISSN 0366-6581.
- ↑ 8.0 8.1 Bigwood J.; Ott J. (1977). "DMT: the fifteen minute trip". Head. 2 (4): 56–61. Archived from the original on January 27, 2006. Retrieved November 28, 2010.
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 Strassman, R. J.; Qualls, C. R.; Uhlenhuth, E. H.; Kellner, R. (1994). "Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale" (PDF). Archives of General Psychiatry. 51 (2): 98–108. doi:10.1001/archpsyc.1994.03950020022002. eISSN 1538-3636. ISSN 2168-622X. PMID 8297217.
- ↑ 10.0 10.1 10.2 Ott, Jonathan (1996). Pharmacotheon: Entheogenic Drugs, Their Plant Sources and History (2nd, densified ed.). Kennewick, WA: Natural Products. ISBN 978-0-9614234-9-0.
- ↑ Pachter I. J.; Zacharias D. E.; Ribeiro O. (1959). "Indole alkaloids of Acer saccharinum (the silver maple), Dictyoloma incanescens, Piptadenia colubrina, and Mimosa hostilis". The Journal of Organic Chemistry. 24 (9): 1285–87. doi:10.1021/jo01091a032. eISSN 1520-6904. ISSN 0022-3263.
- ↑ Fish M. S.; Johnson N. M.; Horning E. C. (1955). "Piptadenia alkaloids. Indole bases of P. peregrina (L.) Benth. and related species". Journal of the American Chemical Society. 72 (22): 5892–95. doi:10.1021/ja01627a034. eISSN 1520-5126. ISSN 0002-7863.
- ↑ Cimino G.; De Stefano S. (1978). "Chemistry of Mediterranean gorgonians: simple indole derivatives from Paramuricea chamaeleon". Comparative Biochemistry and Physiology C. 61 (2): 361–2. doi:10.1016/0306-4492(78)90070-9.
- ↑ Erowid DMT Vault : Profiles of Psychedelic Drugs - DMT
- ↑ Fontanilla, D.; Johannessen, M.; Hajipour, A. R.; Cozzi, N. V.; Jackson, M. B.; Ruoho, A. E. (2009). "The Hallucinogen N,N-Dimethyltryptamine (DMT) Is an Endogenous Sigma-1 Receptor Regulator". Science. 323 (5916): 934–937. doi:10.1126/science.1166127. eISSN 1095-9203. ISSN 0036-8075. OCLC 1644869. PMC 2947205
 . PMID 19213917.
. PMID 19213917.
- ↑ Strassman, Rick J. (1995). "Human psychopharmacology of N,N-dimethyltryptamine". Behavioural Brain Research. 73 (1-2): 121–124. doi:10.1016/0166-4328(96)00081-2. eISSN 1872-7549. ISSN 0166-4328. OCLC 06183451.
- ↑ https://www.researchgate.net/figure/Representative-pharmacokinetic-profiles-of-plasma-alkaloids-over-time-from-the-same_fig7_12895750
- ↑ https://www.dmt-nexus.me/forum/default.aspx?g=posts&m=11514&#post11514
- ↑ https://www.erowid.org/library/books_online/tihkal/tihkal06.shtml
- ↑ https://wiki.dmt-nexus.me/Ingestion_Methods
- ↑ https://www.erowid.org/plants/mimosa/mimosa_info2.shtml
- ↑ https://www.dmt-nexus.me/forum/default.aspx?g=posts&t=10624&p=1
- ↑ https://www.reddit.com/r/DMT/comments/1726ldy/did_anyone_tried_dmt_boof/kol2qu0/?context=3
- ↑ Gallimore, Andrew R.; Strassman, Rick J. (2016). "A Model for the Application of Target-Controlled Intravenous Infusion for a Prolonged Immersive DMT Psychedelic Experience". Frontiers in Pharmacology. 7 (211). doi:10.3389/fphar.2016.00211. eISSN 1663-9812. PMC 4944667
 . PMID 27471468.
. PMID 27471468.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedhttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC4773875/ - ↑ 26.0 26.1 Morales-Garcia, JA; Calleja-Conde, J; Lopez-Moreno, JA; Alonso-Gil, S; Sanz-SanCristobal, M; Riba, J; Perez-Castillo, A (28 September 2020). "N,N-dimethyltryptamine compound found in the hallucinogenic tea ayahuasca, regulates adult neurogenesis in vitro and in vivo". Translational psychiatry. 10 (1): 331. doi:10.1038/s41398-020-01011-0. PMID 32989216.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid=29898390 - ↑ 28.0 28.1 Timmermann, C., Roseman, L., Williams, L., Erritzoe, D., Martial, C., Cassol, H., Laureys, S., Nutt, D., Carhart-Harris, R. (15 August 2018). "DMT Models the Near-Death Experience". Frontiers in Psychology. 9: 1424. doi:10.3389/fpsyg.2018.01424. ISSN 1664-1078.
- ↑ 29.0 29.1 Martial, C; Cassol, H; Charland-Verville, V; Pallavicini, C; Sanz, C; Zamberlan, F; Vivot, RM; Erowid, F; Erowid, E; Laureys, S; Greyson, B; Tagliazucchi, E (March 2019). "Neurochemical models of near-death experiences: A large-scale study based on the semantic similarity of written reports". Consciousness and cognition. 69: 52–69. doi:10.1016/j.concog.2019.01.011. PMID 30711788.
- ↑ Strassman, Rick J. (2001). DMT: The Spirit Molecule. A Doctor's Revolutionary Research into the Biology of Near-Death and Mystical Experiences. Rochester, Vt: Park Street. ISBN 978-0-89281-927-0. OCLC 45195642. ("Chapter summaries". Retrieved 27 February 2012.)
- ↑ Nichols, David E. (2018). "N,N-dimethyltryptamine and the pineal gland: Separating fact from myth". Journal of Psychopharmacology. doi:10.1177/0269881117736919. eISSN 1461-7285. ISSN 0269-8811. OCLC 19962867. PMID 29095071.
- ↑ "'Mystical' psychedelic compound found in normal brains". Neuroscience News. June 27, 2019. Retrieved January 8, 2020.
- ↑ Ly, Calvin; Greb, Alexandra C.; Cameron, Lindsay P.; Wong, Jonathan M.; Barragan, Eden V.; Wilson, Paige C.; Burbach, Kyle F.; Soltanzadeh Zarandi, Sina; Sood, Alexander; Paddy, Michael R.; Duim, Whitney C.; Dennis, Megan Y.; McAllister, A. Kimberley; Ori-McKenney, Kassandra M.; Gray, John A.; Olson, David E. (2018). "Psychedelics Promote Structural and Functional Neural Plasticity". Cell Reports. 23 (11): 3170–3182. doi:10.1016/j.celrep.2018.05.022. ISSN 2211-1247. PMID 29898390. PMC 6082376.
- ↑ Talaie, H.; Panahandeh, R.; Fayaznouri, M. R.; Asadi, Z.; Abdollahi, M. (2009). "Dose-independent occurrence of seizure with tramadol". Journal of Medical Toxicology. 5 (2): 63–67. doi:10.1007/BF03161089. ISSN 1556-9039.
- ↑ "Convention On Psychotropic Substances, 1971" (PDF). United Nations Office on Drugs and Crime. Retrieved January 3, 2020.
- ↑ Herbert Schaepe (International Narcotics Control Board) (January 17, 2001). "International control of the preparation "ayahuasca"". Erowid. Retrieved January 8, 2020.
- ↑ "Poisons Standard December 2019". Federal Register of Legislation. Office of Parliamentary Counsel. November 14, 2019. Retrieved January 8, 2020.
- ↑ "Poisons Act 1964" (PDF). State Law Publisher. July 2, 2014. Archived from the original (PDF) on December 22, 2015.
- ↑ "Misuse of Drugs Act 1981" (PDF). State Law Publisher. Archived from the original (PDF) on December 22, 2015.
- ↑ "Consultation on implementation of model drug schedules for Commonwealth serious drug offenses". Australian Government, Attorney-General's Department. June 24, 2010. Archived from the original on 7 November 2011.
- ↑ "Aussie DMT Ban". American Herb Association Quarterly Newsletter. Vol. 27 (3). August 2012. p. 14.
- ↑ "Wetgeving rond LSD en tripmiddelen" (in Dutch). Druglijn. Retrieved October 23, 2020.
- ↑ "RESOLUÇÃO DA DIRETORIA COLEGIADA - RDC N° 130, DE 2 DE DEZEMBRO DE 2016" (in Portuguese). Agência Nacional de Vigilância Sanitária [National Sanitary Surveillance Agency]. December 5, 2016. p. 22. Retrieved January 8, 2020.
- ↑ "Schedule III". Controlled Drugs and Substances Act (S.C. 1996, c. 19). Government of Canada. Retrieved January 1, 2020.
- ↑ "Bekendtgørelse om euforiserende stoffer - ni nye stoffer tilføjet" (in Danish). Danish Medicines Ageny. August 31, 2015. Retrieved January 1, 2020.
- ↑ "Anlage I BtMG" (in German). Bundesministerium der Justiz und für Verbraucherschutz [Federal Ministry of Justice and Consumer Protection]. Retrieved December 10, 2019.
- ↑ "Sechste Verordnung über die den Betäubungsmitteln gleichgestellten Stoffe" (PDF). Bundesgesetzblatt Jahrgang 1974 Teil I Nr. 6 (in German). Bundesanzeiger Verlag. January 23, 1974. pp. 97–98. Retrieved January 7, 2020.
- ↑ "§ 29 BtMG" (in German). Bundesministerium der Justiz und für Verbraucherschutz [Federal Ministry of Justice and Consumer Protection]. Retrieved December 10, 2019.
- ↑ "Schedule: Controlled Drugs". Misuse of Drugs Act, 1977. Government of Ireland. Retrieved October 23, 2020.
- ↑ Senyor, Eli (August 6, 2013). "Judge's son arrested for importing 2kg of hallucinogenic drug". Ynetnews. Tel Aviv: Yediot Ahronot. Retrieved August 11, 2017.
- ↑ Dr. G. Shreekumar Menon. "The God Drug - DMT". Mangalore Today. Retrieved October 23, 2020.
- ↑ "Tabella I" (PDF) (in Italian). Ministero della Salute [Ministry of Health]. p. 8. Archived from the original (PDF) on July 13, 2019. Retrieved January 7, 2020.
- ↑ "Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem" (in Latvian). VSIA Latvijas Vēstnesis. November 10, 2005. Retrieved January 1, 2020.
- ↑ "Opiumwet" (in Dutch). Ministerie van Binnenlandse Zaken en Koninkrijksrelaties [Ministry of the Interior and Kingdom Relations]. January 1, 2020. Retrieved January 8, 2020.
- ↑ "Schedule 1 Class A controlled drugs". "Reprint as at 13 August 2019: Misuse of Drugs Act 1975". Parliamentary Counsel Office. Retrieved January 7, 2020.
- ↑ "Постановление Правительства РФ от 30.06.1998 N 681 "Об утверждении перечня наркотических средств, психотропных веществ и их прекурсоров, подлежащих контролю в Российской Федерации" (с изменениями и дополнениями)" (in Russian). ГАРАНТ [GARANT]. Retrieved January 8, 2020.
- ↑ "Läkemedelsverkets författningssamling" (PDF) (in Swedish). Christina Rångemark Åkerman (Läkemedelsverket [Swedish Medical Products Agency]). September 21, 2011. p. 12. ISSN 1101-5225. Archived from the original (PDF) on July 22, 2019.
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
- ↑ "Part I: Class A Drugs". "Misuse of Drugs Act 1971". UK Government. Retrieved January 7, 2020.
- ↑ Controlled Substances (PDF), US DOJ, 2022
- ↑ info@aion.cz, A. C.-, 463/2013 Sb. Nařízení vlády o seznamech návykových látek