2C-T-2
| Summary sheet: 2C-T-2 |
| 2C-T-2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common names | 2C-T-2, Rosy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substitutive name | 4-Ethylthio-2,5-dimethoxyphenethylamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Systematic name | 2-[4-(Ethylthio)-2,5-dimethoxyphenyl]ethanamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Psychoactive class | Psychedelic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical class | Phenethylamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2,5-Dimethoxy-4-ethylthiophenethylamine (also known as 2C-T-2, and Rosy) is a psychedelic substance of the phenethylamine chemical class that produces psychedelic effects when administered.
It is a member of the 2C-x family of psychedelic phenethylamines, all of which were derived from the systematic modification of the mescaline molecule.
2C-T-2 was first synthesized and tested for activity in humans by Alexander Shulgin in 1981[1] and described in his 1991 book PiHKAL ("Phenethylamines I Have Known and Loved").[2] It is a member of the so-called "magical half-dozen" which refers to Shulgin's self-rated most important phenethylamine-derived compounds, all of which except mescaline he developed and synthesized himself. They are found within the first book of PiHKAL, and are as follows: mescaline, DOM, 2C-B, 2C-E, 2C-T-2 and 2C-T-7.[3]
Anecdotal reports generally characterize 2C-T-2 as a highly dose sensitive psychedelic known for its open headspace and unpredictable body load.
Very little data exists about the pharmacological properties, metabolism, and toxicity of 2C-T-2, and it has a relatively short history of human usage. Many reports also indicate that its physical effects may be too severe for those who are not already experienced with psychedelics or suffer from pre-existing physical conditions. It is highly advised to approach this hallucinogenic substance with the proper amount of precaution and harm reduction practices if using it.
History and culture
This History and culture section is a stub. As a result, it may contain incomplete or wrong information. You can help by expanding it. |
Following the initial positive results found by Shulgin's research group, a more formal study was carried out by psychedelic psychotherapy pioneer Myron J. Stolaroff who was interested in evaluating the potential use of 2C-T-2 in psychotherapy.[4] Based on the experiences of forty participants in the study who took 2C-T-2, Stolaroff compared the effects favorably to MDMA, describing it as more emotionally opening and permitting a wider exploration of feelings and thoughts.
Chemistry
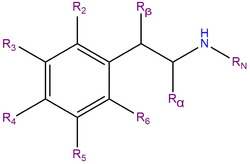
2C-T-2, or 2,5-dimethoxy-4-ethylthiophenethylamine, is a substituted phenethylamine featuring a phenyl ring bound to an amino (NH2) group through an ethyl chain. 2C-T-2 belongs to the 2C family of phenethylamines which contain methoxy functional groups CH3O- attached to carbons R2 and R5 of the benzene ring. 2C-T-2 is also substituted at R4 with an ethyl thioether group.
2C-T-2 is a close structural analogue of 2C-T-7; the two differ by the length of the alkane chain in their thioether functional group.[2]
Pharmacology
The mechanism of action that produces 2C-T-2’s hallucinogenic and entheogenic effects has not been established in scientific literature; however, its primary psychedelic effects are more than likely a result of its efficacy at the 5-HT2A receptor as a partial agonist. This mechanism of action is shared by many other psychedelic phenethylamines and tryptamines.
Athough no established scientific experiments have been performed to establish MAO-A inhibition of 2C-T-2, phenethylamine derivatives substituted with an alkylthio group at the 4 position (4-MTA, and the 2,5-desmethoxy derivative of 2C-T-7) are known to act as selective monoamine oxidase A inhibitors.[citation needed] Furthermore, many compounds of the amphetamine-analogs of the 2C-T-x have been found to have highly selective MAO-A inhibition[5]. Therefore, this substance may likewise have MAOI effects.[6]
However, the role of these interactions and how they result in the psychedelic experience continues to remain elusive.
Subjective effects
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Stimulation - 2C-T-2 is usually considered to be very energetic and stimulating in a fashion that is quite comparable to that of other phenethylamines such as 2C-B, 2C-E and 2C-P.
- Spontaneous physical sensations - The "body high" of 2C-T-2 can be described as very intense and uncomfortable in comparison to 2C-E or 2C-B. In high doses, it's capable of causing painful cramping. It is similar yet distinct from the "body high" experienced on 2C-T-7, but is considered much more unpleasant. The sensation itself can be described as intense and may manifest itself in the form of a continuously shifting tingling sensation that travels up and down the body in spontaneous waves.
- Bodily control enhancement - Although this component is capable of manifesting itself in a distinct and noticeable fashion for most users, it does not generally seem to be as apparent or intense as the same component found within LSD and 2C-B.
- Tactile enhancement
- Nausea - Extreme nausea is commonly reported when consumed in moderate to high doses and either passes once the tripper has vomited or gradually fades by itself as the peak sets in. It can be described as very painful and violent in comparison to 2C-T-7 or 2C-E.
- Stomach cramps
- Vasoconstriction
- Diarrhea
- Temperature regulation suppression
- Increased heart rate
- Pupil dilation
Visual effects 
-
Enhancements
Distortions
- Drifting (melting, flowing, breathing and morphing) - In comparison to other psychedelics, this effect can be described as highly detailed, slow and smooth in motion, static in appearance and extremely realistic in style but with a subtle digital/cartoon-like form.
- Tracers
- After images
- Symmetrical texture repetition
- Colour shifting
- Scenery slicing
Geometry
The visual geometry that is present throughout this experience can be described as somewhat similar in appearance to that of ayahuasca, psilocin, and 4-AcO-DMT with mystical and shamanic undertones which combine with synthetic digital undertones reminiscent of LSD or 2C-E. 2C-T-2's geometry can be comprehensively described through its variations as intricate in complexity, abstract in form, organic but somewhat synthetic in style, structured in organization, brightly lit and multicoloured in scheme, glossy in shading, rounded in edges, large in size, fast in speed, smooth in motion, mostly angular in corners, immersive in-depth and consistent in intensity. The visuals have a contradictory natural and synthetic feel to them which is reminiscent of DOC or 2C-P. Higher doses are more likely to result in states of level 8B visual geometry, but may also lead into level 8A visual geometry (as with 2C-T-7).
Hallucinatory states
At common to high doses, 2C-T-2 is capable of producing a full range of high-level hallucinatory states in a fashion that is more consistent and reproducible than that of many other commonly used psychedelics. This holds particularly true in comparison to other substances within the phenethylamines family. These effects include:
- Transformations
- External hallucination (autonomous entities; settings, sceneries, and landscapes; perspective hallucinations and scenarios and plots) - In comparison to other psychedelics such as ayahuasca, 2C-T-2 is very high in external hallucinations. This particular effect commonly contains hallucinations with scenarios, settings, concepts and autonomous entity contact manifested as dense solidified geometric matter or physical objects of imagined concepts. They are more common within dark environments and can be described as external in their manifestation, lucid in believability, interactive in style and almost exclusively of a personal, religious, spiritual, science-fiction, fantasy, surreal, nonsensical or transcendental nature in their overall theme.
- Internal hallucination (autonomous entities; settings, sceneries, and landscapes; perspective hallucinations and scenarios and plots) - In comparison to other psychedelics such as LSD, 2C-T-2 is very high in hallucinations embedded within visual geometry. This particular effect commonly contains hallucinations with scenarios, settings, concepts and autonomous entity contact. They are more common within dark environments and can be described as internal in their manifestation, lucid in believability, interactive in style and almost exclusively of a personal, religious, spiritual, science-fiction, fantasy, surreal, nonsensical or transcendental nature in their overall theme.
Cognitive effects 
-
The head space of 2C-T-2 is described by many as one which is both insightful and relatively normal in its thought processes even at moderate to high doses.
- Emotion enhancement
- Novelty enhancement
- Time distortion
- Analysis enhancement - This effect is consistent in its manifestation and is outrospection dominant.
- Personal bias suppression
- Conceptual thinking
- Increased music appreciation
- Increased sense of humor
- Immersion enhancement
- Memory suppression
- Thought acceleration
- Thought loops
- Delusion
- Wakefulness
Auditory effects 
Experience reports
There are currently no anecdotal reports which describe the effects of this compound within our experience index. Additional experience reports can be found here:
Toxicity and harm potential
 |
This toxicity and harm potential section is a stub. As a result, it may contain incomplete or even dangerously wrong information! You can help by expanding upon or correcting it. |
The toxicity and long-term health effects of recreational 2C-T-2 use do not seem to have been studied in any scientific context and the exact toxic dosage is unknown.
Anecdotal reports suggest that there are no negative health effects attributed to trying this drug, but nothing can be completely guaranteed.
It is strongly recommended that one use harm reduction practices when using this substance.
Dependence and abuse potential
2C-T-2 is not habit-forming, and the desire to use it can actually decrease with use. It is most often self-regulating.
Tolerance to the effects of 2C-T-2 is built almost immediately after ingestion. After that, it takes about 3 days for the tolerance to be reduced to half and 7 days to be back at baseline (in the absence of further consumption). 2C-T-2 presents cross-tolerance with all psychedelics, meaning that after the consumption of 2C-T-2 all psychedelics will have a reduced effect.
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
If 2C-T-2 does have MAOI effects as has been speculated,[citation needed] this could indicate that 2C-T-2 is more likely to induce serotonin syndrome or general neurotransmitter overload (especially at high dosages) than other serotonergic psychedelics.[5] This may make it dangerous to combine it with other MAOIs, stimulants and certain substances which promotes the release of neurotransmitters such as serotonin or dopamine. These substances include but are not limited to:
Legal status
In November 2003, the European Council decided that 2C-T-2 shall be subjected by the Member States to control measures and criminal penalties within three months.[7]
- Australia: 2C-T-2 is illegal in Australia as of 2005.[citation needed]
- Austria: 2C-T-2 is illegal to possess, produce and sell under the SMG (Suchtmittelgesetz Österreich).[citation needed]
- Belgium: 2C-T-2 was added to the list of illegal psychotropic substances on Nov 8, 2004.[citation needed]
- Brazil: 2C-T-2 is illegal to possess, produce and sell as it is listed on Portaria SVS/MS nº 344.[8]
- Canada: 2C-T-2 would be considered Schedule III as it is a derivative of 2,5-dimethoxyphenethylamine.[9]
- China: As of October 2015, 2C-T-2 is a controlled substance in China.[10]
- Denmark: 2C-T-2 was added to category B of the controlled substances list on August 23, 2003.[11][verification needed]
- Germany: 2C-T-2 is controlled under Anlage I BtMG (Narcotics Act, Schedule I)[12] as of October 10, 1998.[13] It is illegal to manufacture, possess, import, export, buy, sell, procure or dispense it without a license.[14]
- Italy: 2C-T-2 was added to the "Tabella 1" in a Jan 11, 2005 Ministry of Health statement.[citation needed]
- Japan: 2C-T-2 is controlled as a "Designated Substance" (Shitei-Yakubutsu) by the Pharmaceutical Affairs Law, making it illegal to possess or sell.[citation needed]
- Latvia: 2C-T-2 is a Schedule I controlled substance.[15]
- The Netherlands: 2C-T-2 was classified as an unregistered pharmaceutical as of April 12, 1999. Unlicensed manufacture, sale, import, trade and possession of this substance can be prosecuted.[citation needed]
- Switzerland: 2C-T-2 is a controlled substance specifically named under Verzeichnis D.[16]
- United Kingdom: 2C-T-2 is a Class A drug in the United Kingdom as a result of the phenethylamine catch-all clause.[17]
- United States: 2C-T-2 is a Schedule 1 drug in the U.S., making it illegal to possess, manufacture, or import.[citation needed]
See also
External links
Discussion
References
- ↑ Alexander Shulgin (1982). Pharmacology Notes IV (The Shulgin Lab Books) (PDF). Lafayette, CA, USA: Erowid. p. 474.
- ↑ 2.0 2.1 Alexander Shulgin; Ann Shulgin (1991). "#40. 2C-T-2". PiHKAL: A Chemical Love Story. United States: Transform Press. ISBN 0963009605. OCLC 1166889264.
- ↑ Alexander Shulgin; Ann Shulgin (1991). PiHKAL: A Chemical Love Story. United States: Transform Press. ISBN 0963009605. OCLC 1166889264.
- ↑ Stolaroff, M. J.; Wells, C. (1993). "Preliminary results with new psychoactive agents 2C-T-2 and 2C-T-7" (PDF). In Rätsch, C.; Baker, J. Jahrbuch für Ethnomedizin und Bewußtseinsforschung [Yearbook of Ethnomedicine and the Study of Consciousness]. 2. Multidisciplinary Association for Psychedelic Studies (MAPS). pp. 99–117.
- ↑ 5.0 5.1 Gallardo-Godoy, A.; Fierro, A.; McLean, T. H.; Castillo, M.; Cassels, B. K.; Reyes-Parada, M.; Nichols, D. E. (2005). "Sulfur-Substituted α-Alkyl Phenethylamines as Selective and Reversible MAO-A Inhibitors: Biological Activities, CoMFA Analysis, and Active Site Modeling". Journal of Medicinal Chemistry. 48 (7): 2407–2419. doi:10.1021/jm0493109. eISSN 1520-4804. ISSN 0022-2623. OCLC 39480771. PMID 15801832.
- ↑ Theobald, D. S.; Maurer, H. H. (2007). "Identification of monoamine oxidase and cytochrome P450 isoenzymes involved in the deamination of phenethylamine-derived designer drugs (2C-series)". Biochemical Pharmacology. 73 (2): 287–297. doi:10.1016/j.bcp.2006.09.022. eISSN 1873-2968. ISSN 0006-2952. OCLC 01536391. PMID 17067556.
- ↑ "COUNCIL DECISION 2003/847/JHA". Official Journal of the European Union. Office for Official Publications of the European Communites (published December 6, 2003). November 27, 2003. pp. 64–65. OCLC 52224955. L 321.
- ↑ "RESOLUÇÃO DA DIRETORIA COLEGIADA - RDC N° 130, DE 2 DE DEZEMBRO DE 2016" (in Portuguese). Agência Nacional de Vigilância Sanitária (ANVISA) [Brazilian Health Regulatory Agency (ANVISA)]. December 5, 2016.
- ↑ "Schedule III". Controlled Drugs and Substances Act (CDSA). Isomer Design. Retrieved October 10, 2020.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). 国家食品药品监督管理总局 [China Food and Drug Administration (CFDA)]. September 27, 2015. Archived from the original on September 6, 2017.
- ↑ "I Nimekiri" (PDF) (in Estonian). p. 1.
- ↑ "Gesetz über den Verkehr mit Betäubungsmitteln: Anlage I" (in German). Bundesamt für Justiz [Federal Office of Justice]. Retrieved December 10, 2019.
- ↑ "Zwölfte Verordnung zur Änderung betäubungsmittelrechtlicher Vorschriften" (PDF). Bundesgesetzblatt Jahrgang 1998 Teil I Nr. 68 (in German). Bundesanzeiger Verlag (published October 9, 1998). October 7, 1998. p. 3126. ISSN 0341-1095. OCLC 231871244.
- ↑ "Gesetz über den Verkehr mit Betäubungsmitteln: § 29" (in German). Bundesamt für Justiz [Federal Office of Justice]. Retrieved December 10, 2019.
- ↑ "Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem" (in Latvian). VSIA Latvijas Vēstnesis. November 10, 2005. Retrieved January 1, 2020.
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
- ↑ "Schedule 2: Part I: Class A Drugs". "Misuse of Drugs Act 1971". UK Government. Retrieved August 20, 2020.