Pregabalin

Fatal overdose may occur when gabapentinoids are combined with other depressants such as opiates, benzodiazepines, barbiturates, thienodiazepines, alcohol or other GABAergic substances.[1]
It is strongly discouraged to combine these substances, particularly in common to heavy doses.
| Summary sheet: Pregabalin |
| Pregabalin | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common names | Pregabalin, Lyrica, Nervalin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substitutive name | 3-Isobutyl GABA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Systematic name | (S)-3-(Aminomethyl)-5-methylhexanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Psychoactive class | Depressant | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical class | Gabapentinoid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxycodone | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SSRI | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MDMA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pregabalin (also known as 3-isobutyl GABA and by the trade-name Lyrica) is a depressant substance of the gabapentinoid class. Pregabalin is a common prescription drug, which is typically used to treat neuropathic pain, anxiety, restless leg syndrome, and as an adjunct drug in the treatment of seizures.[3][4]
Pregabalin has a pharmacological profile comparable to that of gabapentin as they both share similar mechanisms of action and induce similar subjective effects. The advantages pregabalin has over gabapentin include greater bioavailability and potency,[5] as well as a wider variety of accepted medical applications for pregabalin not seen with gabapentin, such as its successful use in the treatment of anxiety, in which the use of gabapentin was not successful, excluding some more severe cases.[6] [7]
Chemistry
Pregabalin is a structural analog of GABA (gamma-aminobutyric acid), with an isobutyl group substituted on the beta carbon of the aminobutyric chain. Pregabalin is similar in structure to other gabapentinoids, such as gabapentin and phenibut. Pregabalin contains a carboxylated chain of hexane called hexanoic acid. This carbon chain is substituted with an amine group through a methyl bridge in (S) conformation at R3 and a methyl group at R5.
Pharmacology
Pharmacodynamics
The pharmacological action of pregabalin is mediated by binding to the α2δ-1 site of voltage-gated calcium channels (VGCC).[8][9] This site has also been referred to as the gabapentin receptor, as it is the target of the related substance gabapentin (also developed by Pfizer). The α2δ-1 site is present in very high amounts in the sensorial neurons projecting from the dorsal root ganglia and peripheral nerve damage upregulates this structural subunit, causing the sensitization that underlies neuropathic pain development and maintenance [10]. By blocking the α2δ-1 subunit of VGCC at the spinal cord, pregabalin provides relief from pain, as it reduces the release of substances like glutamate, substance P, CGRP (Calcitonin Gene-Related Peptide), which are neurotransmitters that take part in modulating and amplifying pain signals. Advantages to pregabalin over gabapentin include higher bioavailability and potency.
Although pregabalin is a chemical derivative of GABA, it displays no activity at any GABA receptors, including GABAA, GABAB and the benzodiazepine site. Pregabalin, despite its GABA backbone, does not appear to alter GABA levels in the brain, so its pharmacological activity is presumed to be unrelated to GABA.[11] Instead, it is its binding to the α2δ-1 site of voltage-gated calcium channels which appears to be the source of its subjective effects. By binding to this site, pregabalin reduces the release of several excitatory neurotransmitters, including glutamate, substance P, acetylcholine and norepinephrine.[12]
Reduction in the release of glutamate and acetylcholine might be the cause of dissociative / deliriant like effects in high doses.[citation needed]
One study has also shown that pregabalin promotes deep sleep, thus enhancing sleep quality. This may be substantial because reductions in slow-wave sleep have been associated with anxiety and fibromyalgia.[13] Also, an independent action of the gabapentin site on the neurogenesis of excitatory synapses has been discovered. The endogenous neurochemical thrombospondin also binds to this site and is important for the generation of new excitatory synapses. Gabapentin and pregabalin, having a high affinity for this site, block this action and result in lower levels of excitatory synapses in animal models.[9]
As pregabalin treats conditions and neurotransmitters associated with overexcitability of the brain (anxiety, epilepsy, neuropathic pain), its modulation results in the sedating (or calming) effects of pregabalin on the nervous system.[citation needed]
Pharmacokinetics
Pregabalin is rapidly absorbed when administered on an empty stomach, with peak plasma concentrations occurring within 1 to 1.5 hours. Pregabalin oral bioavailability is estimated to be greater than or equal to 90%. The rate of pregabalin absorption is decreased when given with food, resulting in delay of approximately 3 hours to reach peak plasma concentrations, with peak levels themselves, decreased by about 25 to 30%.[14] Administration with food, however, has no clinically significant effect on the extent of absorption.[15]
Pregabalin undergoes negligible metabolism in humans. In experiments using nuclear medicine techniques, it was revealed that approximately 98% of the radioactivity recovered in the urine was unchanged pregabalin. The primary metabolite is N-methyl pregabalin.
Pregabalin is eliminated from the systemic circulation primarily by renal excretion as unchanged substance.[16] The elimination half life is 6.3 hours.[17]
Subjective effects
Each individual can have a very different reaction to pregabalin, thus it is essential to start at lower doses to ensure that it does not have any severe adverse effects such as peripheral edema or muscle pain. Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Stimulation & Sedation - Pregabalin produces mild sedation and improves sleep onset latency modestly. Several studies have shown pregabalin improves sleep quality of those who take it for various indications. It is unknown if this effect is carried over to those who take it recreationally.[18][19] However, it is not an overly sedating substance when taken in the daytime.
- Appetite enhancement - This effect is not particularly prominent, but is reported to occur in some people. It can have a synergistic effect when combined with cannabis.
- Pain relief - Pregabalin is effective against certain types of chronic pain, particularly neuropathic pain, but not against acute pain.[20]
- Spontaneous bodily sensations - The general "body high" of pregabalin can be described as a sharp, pleasurable tingling sensation which is location specific to the hands, feet, and head.
- Physical euphoria - This component, while prominent in the experience, is generally not as strong as the cognitive euphoria that can be induced. The sensation itself can be described as feelings of physical comfort, warmth and bliss.
- Tactile enhancement - Although the user’s body may feel numb, the sense of touch can be heightened at the same time.
- Muscle twitching - Somewhat paradoxically, since pregabalin is used as an adjunct treatment for epilepsy, pregabalin, especially in higher doses, can produce muscle spasms.[citation needed] Anecdotally, seizures have been reported in overdose.[citation needed]
- Respiratory depression - While pregabalin may cause respiratory depression, this effect is not as strong as those with opioids and benzodiazepines.[21]
- Muscle relaxation - While the muscle relaxation experienced on pregabalin is not as powerful as that of diazepam or other benzodiazepines, it is still prominent.
- Dizziness - This effect is fairly prevalent at higher doses.
- Perception of bodily lightness - At very high doses, some users report feeling lighter.
- Increased libido or Decreased libido
- Orgasm depression - Some may experience a delayed but stronger orgasm even if they experience an increase in libido.
- Difficulty urinating - This effect is commonly reported by users.
- Frequent urination
- Motor control loss - At higher doses this effect resembles that of benzodiazepines and alcohol. Users report stumbling and bumping in to walls.
- Tactile suppression - This effect can result in the whole body feeling numb, particularly one’s face. At the same time the user’s sense of touch can be enhanced.
- Seizure suppression - Pregabalin is effective at reducing certain types of seizures such as focal seizures and partial seizures.[22][23]
- Pupil dilation
Visual effects 
-
- Colour enhancement
- Visual acuity suppression - At high dosages, pregabalin can induce slightly blurred vision.
- Tracers - This effect can be seen at high doses and is generally quite mild. It generally does not extend past level 2.
- Depth perception distortions - This effect is quite mild and only appears at very high doses.
- Object alteration - Although this effect is rare, it can still occur spontaneously, typically with heavy doses.
- Drifting
- After images and *Frame rate suppression at high / very high doses. These 2 effects together can make your vision feel more 'slow'.
- Double vision- This effect is quite mild and inconsistently appears at high doses.
Hallucinatory states
While pregabalin isn't commonly thought off as a hallucinogenic drug, it still can cause dissociative and even psychotic like effects at higher doses. Sleep deprivation and genetics might play a role into the hallucinatory states of Pregabalin. Pregabalin's hallucinatory states are (but not limited to) :
- Internal hallucination - At high dosages, one may experience dream-like states and hypnagogia.
- External hallucination - This effect is rare and only occurs on heavy doses and/or when the user is sleep deprived. This effect can be delirious / psychotic in nature. It can include : Object activation, Shadow people and Transformations. It may include even non existent people and animals from a distance, but once the user comes closer they may disappear.
Disconnective effects 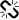
-
- Visual disconnection - This effect is generally quite mild and appears inconsistently at high doses. It results in feeling as if one's sense of vision is distant or vague and being viewed through a screen or window. However, pregabalin is rarely reported to cause holes, spaces and voids or hallucinatory structures.
Cognitive effects 
-
The cognitive effects of pregabalin can be broken down into several components which progressively intensify proportional to dosage. Pregabalin's headspace is comparable to a more clear-headed alcohol or benzodiazepine intoxication, although it can take a more dissociative turn at high dosages.
The most prominent of these cognitive effects generally include:
- Analysis suppression
- Empathy, affection, and sociability enhancement - Pregabalin presents distinct entactogen/empathogenic effects. In contrast to benzodiazepines (which merely increase sociability through disinhibition), at high dosages pregabalin directly increases the urge to communicate with others with feelings of empathy, love, closeness and connection being well-defined. These effects, although weaker than that of MDMA, are still prominent.
- Ego inflation
- Novelty enhancement
- Suggestibility enhancement
- Amnesia - Compared to benzodiazepines, pregabalin is only mildly amnesiac if not combined with other depressants. With chronic usage or high dosages, one should expect more "tip of the tongue" moments and impaired short-term memory (e.g., walking into a room and forgetting what you were supposed to do there). Total blackouts do not seem to occur except in combination with other substances.
- Anxiety suppression
- Emotion enhancement
- Cognitive euphoria[24] - Many users who take pregabalin describe a moderate to even intense euphoria, even at lower doses. Many users describe it as similar to opioid induced euphoria. The sensation itself can be described as powerful and overwhelming feelings of emotional bliss, contentment, and happiness.
- Disinhibition
- Dream potentiation
- Increased music appreciation - The music enhancement from pregabalin can be described as music sounding more detailed, 'higher quality', and even slowed down on higher dosages.
- Motivation enhancement - Like kratom, pregabalin can be mildly sedative yet increase motivation in a stimulant-like fashion.
- Immersion enhancement
- Creativity enhancement - This effect is especially noticeable on higher doses.
- Depersonalization and Derealization - At heavy doses, pregabalin can induce a mild dissociative state. The DPDR from pregabalin can be increased and made more negative (dysphoric) by sleep deprivation.
- Suicidal ideation - In heavy doses, this can lead to suicide attempts.
- Psychosis - Even at therapeutic doses, pregabalin has been shown to have psychotic side effects in a minority of it's users [25][26]. Sleep deprivation strongly potentiates this effect, which may be higher for people genetically predisposed to schizophrenia.
- Mania - When used to treat bipolar disorder [27],it can cause mania, even from therapeutic doses.[28]. This effect typically occurs in a recreational settings, with heavy doses, or when the user is sleep-deprived. It can appear low in intensity (hypomania) or synergize with psychosis. However, this effect is rare, and it's not a part of the typical experience.
- Thought deceleration
Auditory effects 
-
- Auditory enhancement
- Auditory distortion - These are usually mild and only present at extremely high doses.
- Auditory hallucination - At heavier dosages, one may experience tinnitus or the perception of imagined sounds, such as voices.
After effects 
-
The effects during a offset of an Pregabalin experience are generally positive and more similar to an 'afterglow' rather then a 'hangover'. These effects are usually very mild compared to peak effects. The after effects of Pregabalin are:
- Motivation enhancement - Pregabalin is usually mentally stimulating, and the afterglow is similar. Since the high is mostly gone, this can lead to productivity enhancement.
- Increased music appreciation - This effect is not as strong as it would be in the high, but music is noticeably enhanced and sounds more detailed.
- Muscle relaxation - This effect is not as strong as the high but its still here in the afterglow.
- Motor control loss - This effect is weaker than the high but is still noticeable to the user. Driving while in the afterglow / comedown is a bad idea.
- Dizziness - The after effects are mostly positive with pregabalin, but dizziness can still be here, especially after doing a high dose.
Experience reports
Anecdotal reports which describe the effects of this compound within our experience index include:
- Experience:225mg Pregabalin +Cannabis -Bliss and Serenity; a hedonistic evening
- Experience:50mg Pregabalin - Surprisingly strong
- Experience:Pregabalin (2,625 mg, oral) - Pharmaceutical Sunshine
- Experience:Pregabalin (450mg, oral) + Methylphenidate (20mg, oral) - Gaba Flipping
Additional experience reports can be found here:
Medical uses
Pregabalin is used in a medical setting, usually prescribed in capsules, to treat epilepsy, neuropathic pain, fibromyalgia, and generalized anxiety disorder. Its use for epilepsy is as an add-on therapy for partial seizures with or without secondary generalization in adults. Some off-label uses of pregabalin include restless leg syndrome, prevention of migraines, social anxiety disorder, and alcohol withdrawal.
Seizure Prevention
Pregabalin is useful when added to other treatments, when those other treatments are not controlling partial epilepsy. Its use alone is less effective than some other seizure medications. It is unclear how it compares to gabapentin for this use. Pregabalin has also been shown to be effective against alcohol withdrawal induced seizures [29].
Neuropathic pain
The European Federation of Neurological Societies recommends pregabalin as a first line agent for the treatment of pain associated with diabetic neuropathy, post-herpetic neuralgia, and central neuropathic pain. A minority obtain substantial benefit, and a larger number obtain moderate benefit. Other first line agents, including gabapentin and tricyclic antidepressants, are given equal weight as first line agents, and unlike pregabalin, are available as less expensive generics.
Anxiety disorders
The World Federation of Biological Psychiatry recommends pregabalin as one of several first line agents for the treatment of generalized anxiety disorder, but recommends other agents such as SSRIs as first line treatment for obsessive-compulsive disorder and post-traumatic stress disorder. It appears to have anxiolytic effects similar to benzodiazepines with less risk of dependence.
The effects of pregabalin appear after 1 week of use and is similar in effectiveness to lorazepam, alprazolam, and venlafaxine, but pregabalin has demonstrated superiority by producing more consistent therapeutic effects for psychosomatic anxiety symptoms. Long-term trials have shown continued effectiveness without the development of tolerance, and, in addition, unlike benzodiazepines, it has a beneficial effect on sleep and sleep architecture, characterized by the enhancement of slow-wave sleep. It produces less severe cognitive and psychomotor impairment compared to those drugs and may be preferred over the benzodiazepines for these reasons.
Substance disorders
Opioids
Anecdotal reports[30] exist of successful discontinuation of opioid use by supplementing with pregabalin.
Tobacco
One placebo-controlled four-day trial (n=24 completed) investigated the effects of pregabalin on smoking cessation in non-treatment-seeking smokers.[31] This study did not find any statistically significant effect on smoking behavior, although pregabalin reduced some withdrawal symptoms: anxiety, irritability, and frustration. Pregabalin also reduced the measure of subjective "liking" after smoking a cigarette.
Alcohol
A meta-review of five studies concerning the use of pregabalin in treating alcoholism or alcohol withdrawal found positive results for relapse prevention in sober patients at dosages of 150-450 mg/day, but conflicting results for the treatment of acute alcohol withdrawal.[32] Two of the studies concerned the only maintenance of abstinence. Both showed positive results. In one of them (n=59), pregabalin compared favorably to naltrexone on the measure of days abstinent from any amount of alcohol.
Benzodiazepines
One open-label pilot study[33] of 15 individuals with high-dose benzodiazepine dependence reported that all subjects have successfully discontinued benzodiazepines within 14 weeks, while taking supplemental doses of 225-900mg pregabalin/day. The patients also showed reduced anxiety levels and better cognitive functioning. Pregabalin was well tolerated.
Toxicity and harm potential
Pregabalin likely has a low toxicity relative to dose. However, it is potentially lethal when mixed with depressants like alcohol or opioids.
Seizure Risk
Pregabalin has been shown to induce and or increase the risk of seizures at recreational dosages.[34][35][36] There is little evidence showing there is a concern at medical dosages (600mg and under). This risk may be increased when mixed with other drugs that lower seizure threshold. Caution is recommended when using doses of higher than 600mg. Reducing various susceptibility factors may also be beneficial in lowering this risk. Susceptibility factors may include things such as low sodium, lack of sleep, and intensive physical activity.
It is strongly recommended that one use harm reduction practices when using this substance.
Lethal dosage
The LD50 for rodents has been established to be greater than 5000mg/kg. Rat IV LD50 was also determined to be greater than 300mg/kg.[37]
In terms of humans, there exists a case report of a man who ingested 8,400mg pregabalin and eventually fell into a coma but was managed with supportive care alone until he regained consciousness.[38] For comparison, the maximum recommended a therapeutic dose of pregabalin is 600mg/day.[39] Pfizer's official package insert for Lyrica states that the highest accidental ingestion of pregabalin during clinical trials was 8 g, with no significant consequences.[40]
Tolerance and addiction potential
Pregabalin was initially thought to be non-addictive with a low abuse potential and little tolerance development. However, recreational use of the substance has caused a re-evaluation of this assessment. The euphoric effects of the substance and the development of tolerance can lead to the use of dosages far above the therapeutic range, which suggests both the potential for recreational use and addiction.[41][42]
Tolerance will develop to the depressant effects within several months of continuous use. After cessation, the tolerance returns to baseline in 7 - 14 days. Withdrawal symptoms or rebound symptoms are likely to occur after ceasing usage abruptly following a few months or longer of steady dosing and may necessitate a gradual dose reduction.
The withdrawal effects of abrupt cessation of chronic use include anxiety, insomnia, sweating, muscle spasms, gastrointestinal problems, hot and cold flashes, nausea, and a flu-like feeling.
Interactions
One report of a patient entering serotonin syndrome following perioperative Oxycodone and pregabalin exists.[43] However, several studies have failed to find any serotonergic effect whatsoever from pregabalin. One paper states, "Although pregabalin is a structural analog of GABA, it has no clinically significant effects at GABA-A or GABA-B receptors, and it is not converted metabolically into GABA or a GABA agonist. Pregabalin is not a serotonin reuptake inhibitor and does not act as a glutamate receptor antagonist."[44] A more recent study writes that "Pregabalin has no involvement with serotonin and dopamine receptors and does not inhibit dopamine, serotonin, or noradrenaline reuptake."[45] Pregabalin's main mechanism of action is binding and blocking sub receptor on Voltage-Gated Calcium Channels, leading to a downstream reduction of overactive neurons.
If pregabalin has serotonergic effects, it could interact negatively with other serotonergic substances, including SSRIs, MDMA, various analgesics, and possibly other recreational and medical substances. Given the total lack of evidence for any serotonergic activity in multiple studies, it seems possible that the one reported adverse event was a freak accident, caused by unknown factors.
Legal status
Pregabalin is regulated as a prescription drug in most countries.
- Germany: Pregabalin is a prescription medicine, according to Anlage 1 AMVV.[46]
- Norway: Pregabalin is in prescription schedule B alongside most benzodiazepines and painkillers. It was rescheduled to schedule B from the less restrictive schedule C because of reported recreational use, tolerance development and addiction.[47]
- Sweden: Pregabalin is a prescription drug. classified as a controlled substance, as a schedule V drug, since 24 july 2018 [48]
- Switzerland: Pregabalin is listed as a "Abgabekategorie B" pharmaceutical, which requires a prescription.[citation needed]
- Turkey: Pregabalin is a 'green prescription' only substance and illegal when sold or possessed without a prescription.[49][citation needed]
- United Kingdom: The Misuse of Drugs Act 1971 makes it illegal to possess the drug without a prescription and, for such purposes, it is classified as a Class C drug.[50]
- United States: Pregabalin is in Schedule V, indicating "low potential for abuse." For comparison, benzodiazepines are in Schedule IV.[51]
Combinations
with Memantine (anecdotal, N=1)
Add-on of 20mg to 30mg Memantine per day to 75mg Pregabalin is equivalent to 300mg Pregabalin (I had tolerance of that much at the time. I have chronic anxiety)
See also
External links
- Pregabalin (Wikipedia)
- Pregabalin (Erowid Vault)
- Pregabalin (TiHKAL / Isomer Design)
- Pregabalin (Drugs.com)
Literature
- Field, M. J., Cox, P. J., Stott, E., Melrose, H., Offord, J., Su, T., … Williams, D. (2006). Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proceedings of the National Academy of Sciences of the United States of America, 103(46), 17537–42. https://doi.org/10.1073/pnas.0409066103
- Eroglu, Ç., Allen, N. J., Susman, M. W., O’Rourke, N. A., Park, C. Y., Özkan, E., … Barres, B. A. (2009). Gabapentin Receptor α2δ-1 Is a Neuronal Thrombospondin Receptor Responsible for Excitatory CNS Synaptogenesis. Cell, 139(2), 380–392. https://doi.org/10.1016/j.cell.2009.09.025
- Taylor, C. P., Angelotti, T., & Fauman, E. (2007). Pharmacology and mechanism of action of pregabalin: The calcium channel ??2-?? (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Research, 73(2), 137–150. https://doi.org/10.1016/j.eplepsyres.2006.09.008
- Hindmarch, I., Dawson, J., & Stanley, N. (2005). A double-blind study in healthy volunteers to assess the effects on sleep of pregabalin compared with alprazolam and placebo. Sleep, 28(2), 187–93. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16171242
- Wood, D. M., Berry, D. J., Glover, G., Eastwood, J., & Dargan, P. I. (2010). Significant Pregabalin Toxicity Managed with Supportive Care Alone. Journal of Medical Toxicology, 6(4), 435–437. https://doi.org/10.1007/s13181-010-0052-3
- Braga, A. J., & Chidley, K. (2007). Self-poisoning with lamotrigine and pregabalin. Anaesthesia, 62(5), 524–527. https://doi.org/10.1111/j.1365-2044.2006.04913.x
- Kavoussi, R. (2006). Pregabalin: From molecule to medicine. European Neuropsychopharmacology, 16, S128–S133. https://doi.org/10.1016/j.euroneuro.2006.04.005
- Song, H.-K. (2013). Serotonin syndrome with perioperative oxycodone and pregabalin. Pain Physician, 16(October), E632-3. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24077214
- Herman, A. I., Waters, A. J., McKee, S. A., & Sofuoglu, M. (2012). Effects of pregabalin on smoking behavior, withdrawal symptoms, and cognitive performance in smokers. Psychopharmacology, 220(3), 611–617. https://doi.org/10.1007/s00213-011-2507-x
- Guglielmo, R., Martinotti, G., Clerici, M., & Janiri, L. (2012). Pregabalin for alcohol dependence: A critical review of the literature. Advances in Therapy, 29(11), 947–957. https://doi.org/10.1007/s12325-012-0061-5
- Kämmerer, N., Lemenager, T., Grosshans, M., Kiefer, F., & Hermann, D. (2012). [Pregabalin for the reduction of opiate withdrawal symptoms]. Psychiatrische Praxis, 39(7), 351–2. https://doi.org/10.1055/s-0032-1305042
- Bobes, J., Rubio, G., Terán, A., Cervera, G., López-Gómez, V., Vilardaga, I., & Pérez, M. (2012). Pregabalin for the discontinuation of long-term benzodiazepines use: An assessment of its effectiveness in daily clinical practice. European Psychiatry, 27(4), 301–307. https://doi.org/10.1016/j.eurpsy.2010.12.004
- Oulis, P., Konstantakopoulos, G., Kouzoupis, A. V., Masdrakis, V. G., Karakatsanis, N. A., Karapoulios, E., … Papadimitriou, G. N. (2008). Pregabalin in the discontinuation of long-term benzodiazepines’ use. Human Psychopharmacology: Clinical and Experimental, 23(4), 337–340. https://doi.org/10.1002/hup.937
References
- ↑ Risks of Combining Depressants - TripSit
- ↑ Bockbrader, H. N., Radulovic, L. L., Posvar, E. L., Strand, J. C., Alvey, C. W., Busch, J. A., Randinitis, E. J., Corrigan, B. W., Haig, G. M., Boyd, R. A., Wesche, D. L. (August 2010). "Clinical Pharmacokinetics of Pregabalin in Healthy Volunteers". The Journal of Clinical Pharmacology. 50 (8): 941–950. doi:10.1177/0091270009352087. ISSN 0091-2700.
- ↑ EMA (2018), Lyrica
- ↑ Garcia-Borreguero, D., Larrosa, O., Williams, A.-M., Albares, J., Pascual, M., Palacios, J. C., Fernandez, C. (8 June 2010). "Treatment of restless legs syndrome with pregabalin: A double-blind, placebo-controlled study". Neurology. 74 (23): 1897–1904. doi:10.1212/WNL.0b013e3181e1ce73. ISSN 0028-3878.
- ↑ Wesche, D., Bockbrader, H. (March 2005). "A pharmacokinetic comparison of pregabalin and gabapentin". The Journal of Pain. 6 (3): S29. doi:10.1016/j.jpain.2005.01.114. ISSN 1526-5900.
- ↑ Feltner, D. E., Crockatt, J. G., Dubovsky, S. J., Cohn, C. K., Shrivastava, R. K., Targum, S. D., Liu-Dumaw, M., Carter, C. M., Pande, A. C. (June 2003). "A Randomized, Double-Blind, Placebo-Controlled, Fixed-Dose, Multicenter Study of Pregabalin in Patients With Generalized Anxiety Disorder:". Journal of Clinical Psychopharmacology. 23 (3): 240–249. doi:10.1097/01.jcp.0000084032.22282.ff. ISSN 0271-0749.
- ↑ Pande, A. C., Pollack, M. H., Crockatt, J., Greiner, M., Chouinard, G., Lydiard, R. B., Taylor, C. B., Dager, S. R., Shiovitz, T. (August 2000). "Placebo-Controlled Study of Gabapentin Treatment of Panic Disorder:". Journal of Clinical Psychopharmacology. 20 (4): 467–471. doi:10.1097/00004714-200008000-00011. ISSN 0271-0749.
- ↑ Field, M. J., Cox, P. J., Stott, E., Melrose, H., Offord, J., Su, T.-Z., Bramwell, S., Corradini, L., England, S., Winks, J., Kinloch, R. A., Hendrich, J., Dolphin, A. C., Webb, T., Williams, D. (14 November 2006). "Identification of the α 2 -δ-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin". Proceedings of the National Academy of Sciences. 103 (46): 17537–17542. doi:10.1073/pnas.0409066103. ISSN 0027-8424.
- ↑ 9.0 9.1 Eroglu, Ç., Allen, N. J., Susman, M. W., O’Rourke, N. A., Park, C. Y., Özkan, E., Chakraborty, C., Mulinyawe, S. B., Annis, D. S., Huberman, A. D., Green, E. M., Lawler, J., Dolmetsch, R., Garcia, K. C., Smith, S. J., Luo, Z. D., Rosenthal, A., Mosher, D. F., Barres, B. A. (October 2009). "Gabapentin Receptor α2δ-1 Is a Neuronal Thrombospondin Receptor Responsible for Excitatory CNS Synaptogenesis". Cell. 139 (2): 380–392. doi:10.1016/j.cell.2009.09.025. ISSN 0092-8674.
- ↑ https://pmc.ncbi.nlm.nih.gov/articles/PMC1635787/#:~:text=These%20subunits%20have%20distinct%20tissue,and%20maintenance%20are%20not%20clear.
- ↑ Taylor, C. P., Angelotti, T., Fauman, E. (February 2007). "Pharmacology and mechanism of action of pregabalin: The calcium channel α2–δ (alpha2–delta) subunit as a target for antiepileptic drug discovery". Epilepsy Research. 73 (2): 137–150. doi:10.1016/j.eplepsyres.2006.09.008. ISSN 0920-1211.
- ↑ Micheva, Kristina D.; Taylor, Charles P.; Smith, Stephen J. (2006-08-01). "Pregabalin Reduces the Release of Synaptic Vesicles from Cultured Hippocampal Neurons". Molecular Pharmacology. 70 (2): 467–476. doi:10.1124/mol.106.023309. ISSN 1521-0111. PMID 16641316. Retrieved 2023-07-14.
- ↑ Hindmarch, I., Dawson, J., Stanley, N. (February 2005). "A double-blind study in healthy volunteers to assess the effects on sleep of pregabalin compared with alprazolam and placebo". Sleep. 28 (2): 187–193. doi:10.1093/sleep/28.2.187. ISSN 0161-8105.
- ↑ https://www.drugs.com/ppa/pregabalin.html
- ↑ http://web.archive.org/web/20160305012454/http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000546/WC500046602.pdf
- ↑ LYRICA 200 mg, 2016
- ↑ Pregabalin
- ↑ Arnold, L. M., Emir, B., Pauer, L., Resnick, M., Clair, A. (January 2015). "Time to improvement of pain and sleep quality in clinical trials of pregabalin for the treatment of fibromyalgia". Pain Medicine (Malden, Mass.). 16 (1): 176–185. doi:10.1111/pme.12636. ISSN 1526-4637.
- ↑ Perez-Lloret, S., Rojas, G. M., Menoni, M. C., Ruiz, G., Velásquez, C., Rodriguez, H., Rey, M. V., Cardinali, A. D. P., PGB Study Team (February 2012). "Pregabalin beneficial effects on sleep quality or health-related quality of life are poorly correlated with reduction on pain intensity after an 8-week treatment course". Clinical Neuropharmacology. 35 (1): 21–24. doi:10.1097/WNF.0b013e31823df2dc. ISSN 1537-162X.
- ↑ Verma, V., Singh, N., Singh Jaggi, A. (January 2014). "Pregabalin in Neuropathic Pain: Evidences and Possible Mechanisms". Current Neuropharmacology. 12 (1): 44–56. doi:10.2174/1570159X1201140117162802. ISSN 1570-159X.
- ↑ Eipe, N., Penning, J. (2011). "Postoperative respiratory depression associated with pregabalin: A case series and a preoperative decision algorithm". Pain Research & Management : The Journal of the Canadian Pain Society. 16 (5): 353–356. ISSN 1203-6765.
- ↑ Pregabalin
- ↑ Ryvlin, P., Perucca, E., Rheims, S. (December 2008). "Pregabalin for the management of partial epilepsy". Neuropsychiatric Disease and Treatment. 4 (6): 1211–1224. ISSN 1176-6328.
- ↑ Abuse and Misuse Potential of Pregabalin: A Review of the Clinical Evidence. Canadian Agency for Drugs and Technologies in Health. 2012.
- ↑ Olaizola, I., Ellger, T., Young, P., Bösebeck, F., Evers, S., Kellinghaus, C. (1 April 2006). "Pregabalin-associated acute psychosis and epileptiform EEG-changes". Seizure. 15 (3): 208–210. doi:10.1016/j.seizure.2006.02.004. ISSN 1059-1311.
- ↑ Mousailidis, G., Papanna, B., Salmon, A., Sein, A., Al-Hillawi, Q. (28 February 2020). "Pregabalin induced visual hallucinations – a rare adverse reaction". BMC Pharmacology and Toxicology. 21 (1): 16. doi:10.1186/s40360-020-0395-6. ISSN 2050-6511.
- ↑ Schaffer, L. C., Schaffer, C. B., Miller, A. R., Manley, J. L., Piekut, J. A., Nordahl, T. E. (May 2013). "An open trial of pregabalin as an acute and maintenance adjunctive treatment for outpatients with treatment resistant bipolar disorder". Journal of Affective Disorders. 147 (1–3): 407–410. doi:10.1016/j.jad.2012.09.005. ISSN 1573-2517.
- ↑ Yukawa, T., Suzuki, Y., Fukui, N., Otake, M., Sugai, T., Someya, T. (February 2013). "Manic symptoms associated with pregabalin in a patient with conversion disorder". Psychiatry and Clinical Neurosciences. 67 (2): 129–130. doi:10.1111/pcn.12012. ISSN 1323-1316.
- ↑ Becker, H. C., Myrick, H., Veatch, L. M. (1 July 2006). "PREGABALIN IS EFFECTIVE AGAINST BEHAVIORAL AND ELECTROGRAPHIC SEIZURES DURING ALCOHOL WITHDRAWAL". Alcohol and Alcoholism. 41 (4): 399–406. doi:10.1093/alcalc/agl029. ISSN 1464-3502.
- ↑ Kämmerer, N., Lemenager, T., Grosshans, M., Kiefer, F., Hermann, D. (11 June 2012). "Pregabalin zur Reduktion von Opiatentzugssymptomen". Psychiatrische Praxis. 39 (07): 351–352. doi:10.1055/s-0032-1305042. ISSN 0303-4259.
- ↑ Herman, A. I., Waters, A. J., McKee, S. A., Sofuoglu, M. (April 2012). "Effects of pregabalin on smoking behavior, withdrawal symptoms, and cognitive performance in smokers". Psychopharmacology. 220 (3): 611–617. doi:10.1007/s00213-011-2507-x. ISSN 0033-3158.
- ↑ Guglielmo, R., Martinotti, G., Clerici, M., Janiri, L. (November 2012). "Pregabalin for Alcohol Dependence: A Critical Review of the Literature". Advances in Therapy. 29 (11): 947–957. doi:10.1007/s12325-012-0061-5. ISSN 0741-238X.
- ↑ Oulis, P., Konstantakopoulos, G., Kouzoupis, A. V., Masdrakis, V. G., Karakatsanis, N. A., Karapoulios, E., Kontoangelos, K. A., Papadimitriou, G. N. (June 2008). "Pregabalin in the discontinuation of long-term benzodiazepines' use". Human Psychopharmacology: Clinical and Experimental. 23 (4): 337–340. doi:10.1002/hup.937. ISSN 0885-6222.
- ↑ https://doi.org/10.1101/cshperspect.a022863
- ↑ https://doi.org/10.1111/j.0013-9580.2004.455003.x
- ↑ https://doi.org/10.1111/j.0013-9580.2004.455003.x
- ↑ Lyrica material data sheet | http://web.archive.org/web/20161203214718/http://www.pfizer.com/files/products/material_safety_data/722.pdf
- ↑ Wood, D. M., Berry, D. J., Glover, G., Eastwood, J., Dargan, P. I. (December 2010). "Significant Pregabalin Toxicity Managed with Supportive Care Alone". Journal of Medical Toxicology. 6 (4): 435–437. doi:10.1007/s13181-010-0052-3. ISSN 1556-9039.
- ↑ Lyrica (pregabalin) for Fibromyalgia: Uses, Dosage, Side Effects, Interactions, Warnings
- ↑ Lyrica package insert | http://labeling.pfizer.com/ShowLabeling.aspx?id=561#section-10
- ↑ Ja, pregabalin kan misbrukes! - Tidsskrift for Den norske legeforening, 2016
- ↑ "Gabapentin and pregabalin: abuse and addiction". Prescrire International. 21 (128): 152–154. June 2012. ISSN 1167-7422.
- ↑ Song, H.-K. (October 2013). "Serotonin syndrome with perioperative oxycodone and pregabalin". Pain Physician. 16 (5): E632–633. ISSN 2150-1149.
- ↑ Kavoussi, R. (July 2006). "Pregabalin: From molecule to medicine". European Neuropsychopharmacology. 16: S128–S133. doi:10.1016/j.euroneuro.2006.04.005. ISSN 0924-977X.
- ↑ Marks, D. M., Patkar, A. A., Masand, P. S., Pae, C.-U. (June 2009). "Does Pregabalin Have Neuropsychotropic Effects?: A Short Perspective". Psychiatry Investigation. 6 (2): 55–58. doi:10.4306/pi.2009.6.2.55. ISSN 1738-3684.
- ↑ AMVV - Verordnung über die Verschreibungspflicht von Arzneimitteln
- ↑ https://nhi.no/for-helsepersonell/nytt-om-legemidler/pregabalin-lyrica-flyttes-til-reseptgruppe-b/
- ↑ https://lakemedelsverket.se/Alla-nyheter/NYHETER---2018/Pregabalin-narkotikaklassas/
- ↑ YEŞİL REÇETEYE TABİ İLAÇLAR | https://www.titck.gov.tr/storage/Archive/2019/contentFile/01.04.2019%20SKRS%20Ye%C5%9Fil%20Re%C3%A7eteli%20%C4%B0la%C3%A7lar%20Aktif%20SON%20-%20G%C3%9CNCEL_58b1ff4a-2e1c-4867-bad7-eec855d6162a.pdf
- ↑ The Misuse of Drugs Act 1971 (Amendment) Order 2018
- ↑ Title 21 CFR - PART 1308 - Section 1308.15 Schedule V, 2016