Opioids

Fatal overdose may occur when opiates are combined with other depressants such as benzodiazepines, barbiturates, gabapentinoids, thienodiazepines, alcohol or other GABAergic substances.[1]
It is strongly discouraged to combine these substances, particularly in common to heavy doses.
Opioids are a class of psychoactive substances that resemble morphine or other opiates in their pharmacological effects.[citation needed] Opioids work by binding to opioid receptors, which are found in the central and peripheral nervous system and the gastrointestinal tract.[citation needed] The receptors in these organ systems mediate both the beneficial effects and the side effects of opioids.
Although the term opiate is often used as a synonym for opioid, the term opiate is limited to drugs derived from the natural alkaloids found in the resin of the opium poppy (Papaver somniferum).[2] While opioid is a more general term for substances that act primarily on opioid receptors, including natural occurring alkaloids, synthetic substances and opioid peptides.[3]
Opioid dependence can develop with ongoing administration, leading to a withdrawal syndrome with abrupt discontinuation.[4] Opioids are not only well known for their addictive properties, but also for their ability to produce a feeling of euphoria, motivating some to use opioids recreationally.
Chemistry
Opioids are based upon morphine and opium-like structures. They work via their similar chemical structures to the endogenous opioids in the body. Morphine derived opioids, known as morphinans, contain a benzene ring attached to two partially unsaturated cyclohexane rings (phenanthrene) and a 4th nitrogenous ring attached to the core at carbons 9 and 13. There are several classes of opioids which differ greatly in structure from each other. For example, fentanyl and its analogues are structurally unique from morphinans and tramadol derivatives.
Pharmacology
Opioids are known to mimic endogenous endorphins. Endorphins are responsible for analgesia (reducing pain), causing sleepiness, and feelings of pleasure. They can be released in response to pain, strenuous exercise, orgasm, or excitement. This mimicking of natural endorphins results in the drug's euphoric, analgesic (pain relief) and anxiolytic (anti-anxiety) effects.[5]
Receptor types
Opioids act on the three main classes of opioid receptor in the nervous system, μ, κ, δ (mu, kappa, and delta).[6] Each opioid is measured by its agonistic or antagonistic effects towards the receptors, with the responses to the different receptor sub-types (e.g., μ1 and μ2) providing even more effects. Opioid receptors are found mainly within the brain, but also within the spinal cord and digestive tract.[7]
Delta (δ)
The delta receptor is responsible for the analgesia, antidepressant and convulsant effects as well as physical dependence.[6]
Kappa (κ)
The kappa receptor is responsible for the analgesia, anticonvulsant, dissociative and deliriant effects as well as dysphoria, neuroprotection and sedation.[6]
Mu (μ)
The mu receptor is responsible for analgesia, physical dependence, respiratory depression, euphoria, and possible vasodilation.[6]
Agonists of mu opioid receptors produce sedative, euphoric, and anxiolytic effects largely through the interaction of the mu receptors with serotonin, dopamine, and norepinephrine. Activation of mu receptors allows for the disinhibition of serotonin and dopamine neurons by blocking the inhibitory effects of GABA on serotonin and dopamine neurons, thus increasing activity and release of serotonin and dopamine.[8] Mu receptors additionally inhibit the activity of norepinephrine neurons, leading to sedation, anxiolysis, and respiratory depression.[9]
Nociceptin
The nociceptin receptor is responsible for anxiety, depression, appetite and development of tolerance to μ agonists.[10][11]
Zeta (ζ)
The zeta opioid receptor, also known as opioid growth factor receptor (OGFr) is responsible for tissue growth, neural development, and is further implicated in the development in some cancers.[12][13][14][15][16][17] The endogenous ligand for OGFr is met-enkephalin, which is also a powerful endogenous delta opioid receptor agonist.[18]
Subjective effects
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Stimulation or Sedation - At light doses, mu opioid agonists often produce mild to moderate stimulation due to enhancing dopamine and serotonin signaling, which gradually changes to sedation with higher doses due to inhibition of norepinephrine. Opioids with stronger activity at kappa and nociceptin opioid receptors such as fentanyl and morphine tend to be more sedating than opioids which primarily act on mu opioid receptors like kratom and tianeptine
- Respiratory depression - At low to moderate doses, this effect results in the sensation that the breath is slowed down mildly to moderately, but does not cause noticeable impairment. At high doses and overdoses, opioid-induced respiratory depression can result in a shortness of breath, abnormal breathing patterns, semi-consciousness, or unconsciousness. Severe overdoses can result in a coma or death without immediate medical attention.
- Pain relief
- Itchiness
- Constipation
- Cough suppression
- Decreased libido
- Difficulty urinating
- Nausea
- Stomach cramps
- Pupil constriction
- Orgasm suppression
Cognitive effects 
-
- Cognitive euphoria - This can be described as a powerful and overwhelming feeling of emotional bliss, contentment, and happiness.
- Motivation enhancement - Some opioids (such as kratom) are more stimulating than others and seem to enhance motivation.
- Anxiety suppression
- Compulsive redosing
- Dream potentiation
- Increased music appreciation
Visual effects 
-
Suppressions
- Double vision - At high doses, opioids can cause the eyes un-focus and re-focus uncontrollably. This creates a blurred effect and double vision that is present no matter where one focuses their eyes.
Hallucinatory states
- Internal hallucinations - One may experience feelings of hypnagogia during a state of "nodding" which is often accompanied by vivid dream-like visions.
Chemical classes
Naturally occuring
Semi-synthetic
-
- Buprenorphine (Subutex, Suboxone)
- Diacetylmorphine (Heroin)
- Dihydrocodeine
- Desomorphine
- Ethylmorphine
- Hydrocodone
- Hydromorphone
- Oxycodone
- Oxymorphone
- Naloxone (Narcan) - This is a powerful antagonist which precipitates instant withdrawal and is used to recover from overdose.
Nitazenes
-
- α-carboxamido-clonitazene
- α-methyl-metonitazene
- Acetoxynitazene
- Bronitazene
- Butonitazene
- Clonitazene
- Dimetonitazene
- Ethylnitazene
- Ethylthionitazene
- Etodesnitazene (Etazene)
- Etoetonitazene
- Etonitazene
- Etonitazepipne
- Etonitazepyne
- Fluonitazene
- Isotonitazene
- Methylnitazene
- Methylthionitazene
- Metodesnitazene (Metazene)
- meta-Metonitazene
- Metonitazene
- N-desethyl-isotonitazene
- Nitazene
- O-Desmethyl-etonitazene
- Propylnitazene
- Protodesnitazene
- Protonitazene
- t-Butylnitazene
Toxicity and harm potential
 |
This toxicity and harm potential section is a stub. As a result, it may contain incomplete or even dangerously wrong information! You can help by expanding upon or correcting it. |
The short-term non-chronic use of opioids is not associated with any physical or neurological toxicity.[citation needed]
Long term effects
The long-term use of opioids causes hormonal imbalance in both men and women.[19] In men, this opioid-induced androgen deficiency results in abnormally low levels of sex hormones, particularly testosterone.[20]
This negative change in endocrine function in males can lead to: reduced libido, erectile dysfunction, fatigue, depression, reduced facial and body hair, decreased muscle mass, and weight gain.
Another often observed long-term effect is hyperalgesia, an increase in the pain sensitivity of the person. This is specially seen in chronic pain patients on high dose opioid regimes. There is some evidence that NMDA antagonists like ketamine and opoids that are also weak NMDA antagonist such as methadone, levorphanol and tramadol may help delay the onset of hyperalgesia or even revert it.[21]
It is strongly recommended that one use harm reduction practices when using this class of substances.
Tolerance and addiction potential
Due to the highly euphoric nature of these substances, the recreational use and abuse of opioids has an extremely high rate of addiction and dependence. This is combined with a tolerance which builds up quickly, necessitates that the user take increasingly high dosages in order to get the same effects.
The risk of fatal opioid overdoses rise sharply after a period of cessation and relapse, largely because of reduced tolerance.[22] To account for this lack of tolerance, it is safer to only dose a fraction of one's usual dosage if relapsing. It has also been found that the environment one is in can play a role in opioid tolerance. In one scientific study, rats with the same history of heroin administration were significantly more likely to die after receiving their dose in an environment not associated with the drug in contrast to a familiar environment.[23]
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- Alcohol - Both substances potentiate the ataxia and sedation caused by the other and can lead to unexpected loss of consciousness at high doses. Place affected patients in the recovery position to prevent vomit aspiration from excess. Memory blackouts are likely
- Stimulants - Stimulants increase respiration rate which allows for a higher dose of opiates than would otherwise be used. If the stimulant wears off first then the opiate may overcome the user and cause respiratory arrest.
- Benzodiazepines - Central nervous system and/or respiratory-depressant effects may be additively or synergistically present. The two substances potentiate each other strongly and unpredictably, very rapidly leading to unconsciousness. While unconscious, vomit aspiration is a risk if not placed in the recovery position blackouts/memory loss likely.
- DXM - Generally considered to be toxic. CNS depression, difficulty breathing, heart issues, and liver toxicity have been observed. Additionally if one takes DXM, their tolerance of opiates goes down slightly, thus causing additional synergistic effects.
- GHB/GBL - The two substances potentiate each other strongly and unpredictably, very rapidly leading to unconsciousness. While unconscious, vomit aspiration is a risk if not placed in the recovery position
- Ketamine - Both substances bring a risk of vomiting and unconsciousness. If the user falls unconscious while under the influence there is a severe risk of vomit aspiration if they are not placed in the recovery position.
- MAOIs - Coadministration of monoamine oxidase inhibitors (MAOIs) with certain opioids has been associated with rare reports of severe adverse reactions. There appear to be two types of interaction, an excitatory and a depressive one. Symptoms of the excitatory reaction may include agitation, headache, diaphoresis, hyperpyrexia, flushing, shivering, myoclonus, rigidity, tremor, diarrhea, hypertension, tachycardia, seizures, and coma. Death has occurred in some cases.
- MXE - MXE can potentiate the effects of opioids but also increases the risk of respiratory depression and organ toxicity.
- Nitrous - Both substances potentiate the ataxia and sedation caused by the other and can lead to unexpected loss of consciousness at high doses. While unconscious, vomit aspiration is a risk if not placed in the recovery position. Memory blackouts are common.
- PCP - PCP may reduce opioid tolerance, increasing the risk of overdose.
- Tramadol - Increased risk of seizures. Tramadol itself is known to induce seizures and it may have additive effects on seizure threshold with other opioids. Central nervous system- and/or respiratory-depressant effects may be additively or synergistically present.
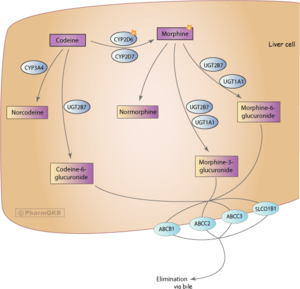
- Grapefruit - While grapefruit is not psychoactive, it may affect the metabolism of certain opioids. Tramadol, oxycodone, and fentanyl are all primarily metabolized by the enzyme CYP3A4, which is potently inhibited by grapefruit juice[24]. This may cause the drug to take longer to clear from the body. it may increase toxicity with repeated doses. Methadone may also be affected[24]. Codeine and hydrocodone are metabolized by CYP2D6. People who are on medicines that inhibit CYP2D6, or that lack the enzyme due to a genetic mutation will not respond to codeine as it can not be metabolized into its active product: morphine.
See also
External links
References
- ↑ Risks of Combining Depressants - TripSit
- ↑ Hemmings, Hugh C.; Egan, Talmage D. (2013). Pharmacology and Physiology for Anesthesia: Foundations and Clinical Application: Expert Consult - Online and Print. Elsevier Health Scienc,es. p. 253. ISBN 1437716792.
Opiate is the older term classically used in pharmacology to mean a drug derived from opium. Opioid, a more modern term, is used to designate all substances, both natural and synthetic, that bind to opioid receptors (including antagonists).
- ↑ Hemmings, Hugh C.; Egan, Talmage D. (2013). Pharmacology and Physiology for Anesthesia: Foundations and Clinical Application: Expert Consult - Online and Print. Elsevier Health Sciences. p. 253. ISBN 1437716792.
Opiate is the older term classically used in pharmacology to mean a drug derived from opium. Opioid, a more modern term, is used to designate all substances, both natural and synthetic, that bind to opioid receptors (including antagonists).
- ↑ Cammarano, W. B., Pittet, J.-F., Weitz, S., Schlobohm, R. M., Marks, J. D. (April 1998). "Acute withdrawal syndrome related to the administration of analgesic and sedative medications in adult intensive care unit patients:". Critical Care Medicine. 26 (4): 676–684. doi:10.1097/00003246-199804000-00015. ISSN 0090-3493.
- ↑ Boecker H, Sprenger T, Spilker ME, Henriksen G, Koppenhoefer M, Wagner KJ, Valet M, Berthele A, Tolle TR (November 2008). "The runner's high: opioidergic mechanisms in the human brain". Cerebral Cortex. 18 (11): 2523–31. doi:10.1093/cercor/bhn013. PMID 18296435.
- ↑ 6.0 6.1 6.2 6.3 Opioid - Chapter 2: The Endogeneous Opioid Systems (http://www.stoppain.org / Beth Israel Medical Center's Department of Pain Medicine and Palliative Care) | https://web.archive.org/web/20110719072413/http://www.stoppain.org/pcd/_pdf/OpioidChapter2.pdf
- ↑ Holzer, P. (5 June 2009). "Opioid receptors in the gastrointestinal tract". Regulatory peptides. 155 (1–3): 11–17. doi:10.1016/j.regpep.2009.03.012. ISSN 0167-0115.
- ↑ https://www.sciencedirect.com/science/article/abs/pii/S1043661818306145
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3274960/
- ↑ Calo' G, Guerrini R, Rizzi A, Salvadori S, Regoli D (April 2000). "Pharmacology of nociceptin and its receptor: a novel therapeutic target". British Journal of Pharmacology. 129 (7): 1261–83. doi:10.1038/sj.bjp.0703219. PMC 1571975
 . PMID 10742280.
. PMID 10742280.
- ↑ Toll L, Bruchas MR, Calo' G, Cox BM, Zaveri NT (April 2016). "Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems". Pharmacological Reviews. 68 (2): 419–57. doi:10.1124/pr.114.009209. PMID 26956246.
- ↑ Zagon IS, Wu Y, McLaughlin PJ (August 1999). "Opioid growth factor and organ development in rat and human embryos". Brain Res. 839 (2): 313–22. doi:10.1016/S0006-8993(99)01753-9. PMID 10519055.
- ↑ Sassani JW, Zagon IS, McLaughlin PJ (May 2003). "Opioid growth factor modulation of corneal epithelium: uppers and downers". Curr. Eye Res. 26 (5): 249–62. doi:10.1076/ceyr.26.4.249.15427. PMID 12854052.
- ↑ Zagon IS, Smith JP, McLaughlin PJ (March 1999). "Human pancreatic cancer cell proliferation in tissue culture is tonically inhibited by opioid growth factor". Int. J. Oncol. 14 (3): 577–84. doi:10.3892/ijo.14.3.577. PMID 10024694.
- ↑ McLaughlin PJ, Levin RJ, Zagon IS (May 1999). "Regulation of human head and neck squamous cell carcinoma growth in tissue culture by opioid growth factor". Int. J. Oncol. 14 (5): 991–8. doi:10.3892/ijo.14.5.991. PMID 10200353.
- ↑ Cheng F, Zagon IS, Verderame MF, McLaughlin PJ (November 2007). "The opioid growth factor (OGF)-OGF receptor axis uses the p16 pathway to inhibit head and neck cancer". Cancer Research. 67 (21): 10511–8. doi:10.1158/0008-5472.CAN-07-1922. PMID 17974995.
- ↑ Donahue RN, McLaughlin PJ, Zagon IS (March 2009). "Cell Proliferation of Human Ovarian Cancer is Regulated by the Opioid Growth Factor - Opioid Growth Factor Receptor Axis". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 296 (6): R1716–25. doi:10.1152/ajpregu.00075.2009. PMID 19297547.
- ↑ Christoph Stein (1999). Opioids in pain control: basic and clinical aspects. Cambridge University Press. pp. 22–23. ISBN 978-0-521-62269-1. Retrieved 25 November 2011.
- ↑ Brennan, M. J. (March 2013). "The effect of opioid therapy on endocrine function". The American Journal of Medicine. 126 (3 Suppl 1): S12–18. doi:10.1016/j.amjmed.2012.12.001. ISSN 1555-7162.
- ↑ Smith, H. S., Elliott, J. A. (July 2012). "Opioid-induced androgen deficiency (OPIAD)". Pain Physician. 15 (3 Suppl): ES145–156. ISSN 2150-1149.
- ↑ Lee, M., Silverman, S. M., Hansen, H., Patel, V. B., Manchikanti, L. (April 2011). "A comprehensive review of opioid-induced hyperalgesia". Pain Physician. 14 (2): 145–161. ISSN 2150-1149.
- ↑ Why Heroin Relapse Often Ends In Death - Lauren F Friedman (Business Insider) | http://www.businessinsider.com.au/philip-seymour-hoffman-overdose-2014-2
- ↑ Siegel, S., Hinson, R. E., Krank, M. D., McCully, J. (23 April 1982). "Heroin "Overdose" Death: Contribution of Drug-Associated Environmental Cues". Science. 216 (4544): 436–437. doi:10.1126/science.7200260. ISSN 0036-8075.
- ↑ 24.0 24.1 Ershad, M., Cruz, M. D., Mostafa, A., Mckeever, R., Vearrier, D., Greenberg, M. I. (March 2020). "Opioid Toxidrome Following Grapefruit Juice Consumption in the Setting of Methadone Maintenance". Journal of Addiction Medicine. 14 (2): 172–174. doi:10.1097/ADM.0000000000000535. ISSN 1932-0620.