Amphetamine
| Summary sheet: Amphetamine |
| Amphetamine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common names | Amphetamine, Speed, Adderall, Pep | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substitutive name | α-Methylphenethylamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Systematic name | (RS)-1-Phenylpropan-2-amine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Psychoactive class | Stimulant | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical class | Phenethylamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alcohol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GHB | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GBL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Opioids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cocaine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cannabis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Caffeine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ketamine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Methoxetamine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Psychedelics | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DXM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PCP | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 25x-NBOMe | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2C-T-x | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5-MeO-xxT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DOx | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tramadol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| aMT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MAOIs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Amphetamine (also known as alpha-methylphenethylamine, amfetamine, speed, or by brand names including Adderall, Dexedrine and Tentin) is a classical stimulant substance of the phenethylamine class. It is the parent compound of the substituted amphetamines, a diverse group that includes methamphetamine, MDMA, cathinone, and bupropion. The mechanism of action involves promoting release of the neurotransmitters dopamine and norepinephrine.[2]
It was first synthesized in 1887, but its psychostimulant effects were not discovered until 1929.[3] In the 1930s, it was sold over-the-counter under the name "Benzedrine" as a decongestant.[4] It became widely used to treat a range of ailments such as alcohol hangover, narcolepsy, depression, and obesity.[5] Due to issues with addiction and abuse, it was eventually listed as a controlled substance under the United Nations 1971 "Convention on Psychotropic Substances".[6]
Amphetamine is now primarily a prescription drug used to treat attention deficit hyperactivity disorder (ADHD), narcolepsy, and obesity.[7][8] Treatment with Amphetamine, among other stimulants, may be associated with neuroprotection for ADHD patients by reducing neurotransmitter deficiencies commonly linked to neurological deficits within untreated subjects with ADHD.[9][10] Additionally, it sees widespread illicit use as a performance enhancing agent and recreational substance.
Subjective effects include stimulation, focus enhancement, motivation enhancement, increased libido, appetite suppression, and euphoria. It is usually taken orally, but can also be insufflated, injected, or administered rectally. Lower doses tend to increase focus and productivity while higher doses tend to increase sociability, sexual desire, and euphoria.
Amphetamine has high abuse potential. Chronic use (i.e. high dose, repeat administration) is associated with compulsive redosing, escalating tolerance, and psychological dependence. Additionally, abuse has been linked to a number of health conditions, especially cardiovascular issues such as high blood pressure and increased risk of stroke.[11]
It is highly advised to use harm reduction practices if using this substance.
History and culture
Amphetamine was first synthesized in Germany in 1887 by the Romanian chemist Lazăr Edeleanu, who named it phenylisopropylamine.[12] However, its stimulant effects remained unknown until 1927, when it was independently re-synthesized by Gordon Alles and discovered to have sympathomimetic properties.[13]
In late 1933, Smith, Kline and French began selling amphetamine in the form of a decongestant inhaler under the name Benzedrine.[4] Benzedrine sulfate was introduced 3 years later and was used to treat a wide variety of medical conditions, including narcolepsy, obesity, low blood pressure, low libido, and chronic pain.[14]
During World War II, amphetamine and methamphetamine were used extensively by both the Allied and Axis forces for their stimulant and performance-enhancing effects.[15][16] As its addictive properties became known, governments began to place strict controls on its sale.[17]
Amphetamine is still illegally synthesized and sold on the black market, primarily in European countries.[18] Among European Union (EU) member states, 1.2 million young adults used illicit amphetamine or methamphetamine in 2013. During 2012, approximately 5.9 metric tons of illicit amphetamine were seized within EU member states;[18] the "street price" of illicit amphetamine within the EU ranged from €6–38 per gram during the same period.[18]
Outside Europe, the illicit market for amphetamine is much smaller than the market for methamphetamine and MDMA.[18]
Chemistry
Amphetamine, also known as alpha-methylphenethylamine, is a synthetic substance of the phenethylamine family. The chemical structure of amphetamine consists of phenethylamine, a phenyl ring bound to an amino (NH2) group through an ethyl chain, with an additional methyl substitution at Rα. The name 'amphetamine' is a contraction from αlphamethylphenethylamine
It is the parent compound of the substituted amphetamines, a highly diverse group that includes such substances as bupropion, phenmetrazine, methamphetamine, MDMA, and the DOx series.
At room temperature, the pure freebase is a mobile, colorless, and volatile liquid with a characteristically strong amine odor, and acrid, burning taste.[19]
Enantiomers
Amphetamine is a chiral compound. Racemic amphetamine (dl-amphetamine) contains two optical isomers, or enantiomers:
- levoamphetamine is the 'left-handed' enantiomer form of amphetamine.
- dextroamphetamine is the 'right-handed' enantiomer form of amphetamine.
Adderall and many other formulations of mixed amphetamine salts contain the enantiomers in a 3:1 ratio of d to l. This is achieved by mixing one part racemic amphetamine and one part dextroamphetamine.
Pharmacology
Amphetamine exerts its behavioural effects by increasing the signaling activity of neurotransmitters norepinephrine and dopamine in the reward and executive function pathways of the brain. The reinforcing and motivational effects of amphetamine are mostly due to enhanced dopaminergic activity in the mesolimbic pathway.[20]
The euphoric and locomotor-stimulating effects of amphetamine are dependent upon the magnitude and speed by which it increases synaptic dopamine and norepinephrine concentrations in the striatum.[3]
It is a potent full agonist of the trace amine-associated receptor 1 (TAAR1) and interacts with vesicular monoamine transporter 2 (VMAT2).[21][22][23] Combined action on TAAR1 and VMAT2 results in increased concentrations of dopamine and norepinephrine in the synapses, which stimulates neuronal activity.
Dextroamphetamine is a more potent agonist of TAAR1 than levoamphetamine.[24] Consequently, dextroamphetamine produces greater CNS stimulation than levoamphetamine, roughly three to four times more, but levoamphetamine has slightly stronger cardiovascular and peripheral effects.[25][26]
The exact bioavailability of amphetamine is not known, but it is believed to be over 75% by mouth, and higher by injection or intranasal administration[27]. Its absorption and excretion may be pH dependent. The basic form is more readily absorbed in the intestine and less readily removed by the kidneys, potentially increasing its half life [27]. It is removed by the kidneys via excretion and a small amount is removed by hepatic enzymes.
Subjective effects
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Stimulation - Amphetamine is reported to be very energetic and stimulating. It can encourage physical activities such as dancing, socializing, running, or cleaning. The particular style of stimulation that amphetamine produces can be described as forced. This means that at higher dosages, it becomes difficult or impossible to keep still. Jaw clenching, involuntary bodily shakes, and vibrations become present, resulting in extreme shaking of the entire body, unsteadiness of the hands, and a general loss of fine motor control. This is replaced with mild fatigue and general exhaustion during the offset of the experience.
- Spontaneous bodily sensations - The "body high" of amphetamine can be described as a moderate euphoric tingling sensation that encompasses the entire body. This sensation maintains a consistent presence that steadily rises with the onset and hits its limit once the peak has been reached.
- Physical euphoria
- Abnormal heartbeat[28]
- Increased heart rate[28]
- Increased blood pressure- By about 30mmHg systolic and 20mmHg diastolic, from naive users taking 40mg d-AMP.[28][29]
- Appetite suppression[30]
- Bronchodilation[31]
- Dehydration[citation needed]
- Dry mouth[32]
- Frequent urination
- Difficulty urinating
- Increased bodily temperature[33]
- Increased perspiration
- Mania - Amphetamine can produce mania in genetically predisposed individuals, such as those on the spectrum of bipolar disorder or schizophrenia. Higher doses and sleep deprivation appears to increase the risk.
- Nausea - This can be mitigated by eating before dosing and throughout the experience.
- Pupil dilation - This effect is experienced only at common to high dosages as well as in low lighting and is more prominent on the comedown.
- Reflex syncope[citation needed] - This may be experienced due to an Effect:Abnormal heartbeat causing a sudden loss in blood pressure.
- Stamina enhancement
- Teeth grinding - Teeth grinding may be present at higher doses. However, it is less intense than that of MDMA.
- Temporary erectile dysfunction
- Vasoconstriction[34] - Amphetamine use causes blood vessels to constrict resulting in not enough blood reaching some parts of the body. This can cause feelings of tingling or pain, a cold feeling, numbness, paleness, or skin color changes especially in the fingers and toes.
Visual effects 
-
The visual effects of amphetamine are inconsistent and occur only mildly noticeable at higher doses. They are somewhat comparable to deliriant visuals and occur more readily in darker areas.
Distortions
- Drifting - This effect is usually subtle and barely noticeable and only occurs at higher dosages or when combined with cannabis. Commonly this consists of level 1-2 drifting.
- Brightness alteration - Amphetamine can make spaces seem brighter as a result of its pupil dilating effects.
- Tracers - This effect is imperceptible with low dosages. It's most pronounced with bigger dosages and especially when someone becomes sleep deprived, what on the other hand can be easily provoked by other effects of this substantion.
It can get up to around 2.5-3 points on the psychonaut wiki scale. (click Traces above to read more)
Hallucinatory states
- Transformations - This effect occurs very rarely, and typically only when the user has taken high doses, is coming down, or has been awake for unusually long periods. They are usually very mild when they do occur.
- Geometry - This effect is reported by some users of amphetamine and related substances, typically at heavier doses when one is attempting to sleep. It can be described in its variations as simplistic, algorithmic, synthetic, dimly lit, multicolored, glossy, sharp edges, zoomed out, smooth, angular, immersive, and progressive. It typically occurs at level 3 however may progress to 4 and 5 when combined with substances like cannabis or DXM
Cognitive effects 
-
The cognitive effects of amphetamine are oftentimes subjectively perceived as being more clearheaded and “functional” compared to other strong stimulants such as cocaine. In low to moderate doses the amphetamine headspace can, in many ways “mimic sobriety”; thus leading users or even onlookers to mistake the effects for more natural or mundane states of stimulation and focus. Individuals diagnosed with ADHD/ADD (dopamine and norepinephrine deficiencies in certain brain regions) may however react starkly differently to amphetamine in low or moderate dosages.
- Analysis enhancement - This effect is considered to be more consistent and prevalent when compared to other common stimulants.
- Cognitive euphoria
- Compulsive redosing- This behavioral effect is generally less pronounced than it is with cocaine or when amphetamine is insufflated as opposed to taken orally.
- Ego inflation
- Emotion suppression - This effect is typically most intense at light and common doses or with frequent usage, and is more commonly reported from medical usage rather than recreational.
- Focus enhancement - This effect is most effective at low to moderate doses, as anything higher will usually impair concentration or cause the user to be too physically active or restless to focus.
- Increased libido - While amphetamine use can cause feelings of enhanced sexual desire, the constricting of blood vessels may make it difficult to achieve or maintain an erection.
- Increased music appreciation
- Irritability - This is more likely to occur at higher doses and/or during the comedown.
- Memory enhancement
- Motivation enhancement - Anecdotally, this component can be seemingly more prevalent when the levoamphetamine enantiomer is present in this substance, such as with racemic amphetamine or mixtures of amphetamine salts such as Adderall.
- Psychosis - This effect only occurs in either predisposed individuals, or after chronic, high frequency use, or due to sleep deprivation. Repeated administration of threshold/very low dosages (such as 2.5mg) however may also be associated with this effect possibly due to the “dopamine hypersensitivity” hypothesis. However this topic is somewhat controversial and speculative.
- Suggestibility suppression
- Thought acceleration
- Thought organization
- Time distortion - This can be described as the experience of time speeding up and passing much quicker than it usually would when sober due to increased levels of dopamine.
- Wakefulness - This component is generally considered to be less intense when compared to methamphetamine but more intense than with cocaine.
After effects 
-
The effects which occur during the offset of a stimulant experience generally feel negative and uncomfortable in comparison to the effects which occurred during its peak. This is often referred to as a "comedown" or “crash” and occurs because of neurotransmitter (specifically catecholamine) depletion. Its effects commonly include:
- Anxiety - Anxiety can reach severe levels during the comedown in some users.
- Appetite suppression
- Cognitive fatigue
- Depression
- Increased heart rate - While blood concentration of amphetamine and most subjective effects are highest about 3 hours after administration, heart rate peaks much later at 10 hours after administration.[29]
- Irritability
- Restless legs
- Sleep paralysis - Some users note sleep paralysis after consuming amphetamine.
- Dream suppression
- Thought deceleration
- Wakefulness - The insomnia following a repeated series of amphetamine doses can last for longer than a day in some users.
- Motivation suppression - Experiences can range from mild demotivation to extreme states of disinterest. This effect is more prominent at common and heavy doses.
Experience reports
There are currently 2 experience reports which describe the effects of this substance in our experience index.
- Experience:15mg Amphetamine (Oral) - Fairly Pleasant and Productive
- Experience:3-MeO-PCP, LSD, Clonazolam, and Amphetamine - Excessive Amounts and Excessive Confusion
Additional experience reports can be found here:
Toxicity and harm potential
 |
This toxicity and harm potential section is a stub. As a result, it may contain incomplete or even dangerously wrong information! You can help by expanding upon or correcting it. |

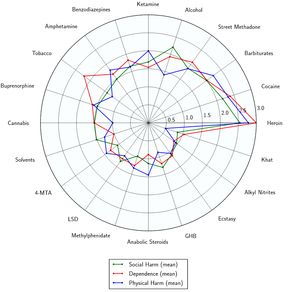
As of March 2014, there is no evidence that amphetamine is directly neurotoxic in humans.[37] However, high-dose amphetamine can cause indirect neurotoxicity as a result of increased oxidative stress from reactive oxygen species and autoxidation of dopamine.[20][38][39]
In rodents and primates, sufficiently high doses of amphetamine causes damage to dopamine neurons, characterized as reduced transporter and receptor function.[40] Animal models of neurotoxicity from high-dose amphetamine exposure indicate that the occurrence of hyperpyrexia (i.e., core body temperature ≥ 40 °C) is necessary for the development of amphetamine-induced neurotoxicity. [41]
Melatonin has been shown to prevent (if used 30min+ before dosing) and reverse amphetamine induced neurotoxicity of TH-pSer40 and calpastatin levels in the Substantia Nigra of rats.[42][43]
It is strongly recommended that one use harm reduction practices when using this substance.
Lethal dosage
The LD50 (the dosage required to kill 50% of the test subjects) of amphetamine in rats has been found to be between roughly 15 mg and 180 mg per kilogram, depending on the study.[44] No formal studies in humans have been carried out and the exact toxic dosage is unknown.
Dependence and abuse potential
Amphetamine has high abuse potential and can cause psychological dependence with chronic use.
When dependence has developed, cravings and withdrawal effects may occur if use is suddenly discontinued.[45][46] Withdrawal symptoms include paranoia, depression, dream potentiation, anxiety, itching, mood swings, irritability, fatigue, insomnia, an intense craving for more amphetamine or other stimulants.
Addiction is a serious risk with chronic or heavy recreational amphetamine use, but is unlikely to arise from typical medical use.[47][48][49]
Tolerance to many of the effects of amphetamine develops with prolonged and repeated use. This results in the user having to administer increasingly large doses to achieve the same effects. Upon single administration, it takes about 3 - 7 days for the tolerance to be reduced to half and 1 - 2 weeks to be back at baseline (in the absence of further consumption).
Amphetamine exhibits cross-tolerance with all dopaminergic stimulants, meaning that after the consumption of amphetamine most stimulants will have a reduced effect.
Psychosis
Severe amphetamine overdose can result in a stimulant psychosis that may present with a variety of symptoms (e.g., paranoia, hallucinations, delusions).[50] A review on treatment for amphetamine abuse-induced psychosis states that about 5–15% of users fail to recover completely.[50][51] The same review asserts that antipsychotic medications effectively resolve the symptoms of acute amphetamine psychosis.[50] Psychosis very rarely arises from therapeutic use.[52]
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- Alcohol - Drinking alcohol on stimulants is considered risky because it reduces the sedative effects of the alcohol that the body uses to gauge drunkenness. This often leads to excessive drinking with greatly reduced inhibitions, increasing the risk of liver damage and increased dehydration. The effects of stimulants will also allow one to drink past a point where they might normally pass out, increasing the risk. If you do decide to do this then you should set a limit of how much you will drink each hour and stick to it, bearing in mind that you will feel the alcohol and the stimulant less.
- GHB/GBL - Stimulants increase respiration rate allowing a higher dose of sedatives. If the stimulant wears off first then the depressant effects of the GHB/GBL may overcome the user and cause respiratory arrest.
- Opioids - Stimulants increase respiration rate allowing a higher dose of opiates. If the stimulant wears off first then the opiate may overcome the patient and cause respiratory arrest.
- Cocaine - The rewarding effects of cocaine are mediated by DAT inhibition, and an increase of exocytosis of dopamine through the cell membrane. Amphetamine reverses the direction of DAT and the direction vesicular transports within the cell by a pH mediated mechanism of displacement, thus excludes the regular mechanism of dopamine release through means of exocytosis because the effects Na+/K+ ATPase are inhibited. You will find cardiac effects with the combination of cocaine and amphetamine due to a SERT mediated mechanism from the subsequent activation of 5-HT2B, which is an effect of serotonin-related valvulopathy. Amphetamines generally cause hypertension in models of abuse, and this combination can increase the chances of syncope due to turbulent blood flow during valve operation. The rewarding mechanisms of cocaine are reversed by administration of amphetamine.[53][54]
- Cannabis - Stimulants increase anxiety levels and the risk of thought loops and paranoia which can lead to negative experiences.
- Caffeine - This combination of stimulants is generally considered unnecessary and may increase strain on the heart, as well as potentially causing anxiety and physical discomfort.
- Tramadol - Tramadol and stimulants both increase the risk of seizures.
- DXM - Both substances raise heart rate, in extreme cases, panic attacks caused by these substances have led to more serious heart issues.
- Ketamine - Combining amphetamine and ketamine may result in psychoses that resemble schizophrenia, but not worse than the psychoses produced by either substance alone, but this is debatable. This is due to amphetamines ability to attenuated the disruption of working memory caused by ketamine. Amphetamine alone may result in grandiosity, paranoia, or somatic delusions with little to no effect on negative symptoms. Ketamine, however, will result in thought disorders, disruption of executive functioning, and delusions due to a modification of conception. These mechanisms are due to an increase of dopaminergic activity in the mesolimbic pathway caused by amphetamine due to its pharmacology effecting dopamine, and due to a disruption of dopaminergic functioning in the mesocortical pathways via NMDA antagonism effects of ketamine. Combining the two, you may expect mainly thought disorder along with positive symptoms.[55]
- PCP - Increases risk of tachycardia, hypertension, and manic states.
- Methoxetamine - Increases risk of tachycardia, hypertension, and manic states.
- Psychedelics (e.g. LSD, mescaline, psilocybin) - Increases risk of anxiety, paranoia, and thought loops.
- 25x-NBOMe - Amphetamines and NBOMes both provide considerable stimulation that when combined they can result in tachycardia, hypertension, vasoconstriction and, in extreme cases, heart failure. The anxiogenic and focusing effects of stimulants are also not good in combination with psychedelics as they can lead to unpleasant thought loops. NBOMes are known to cause seizures and stimulants can increase this risk.
- 2C-T-x - Suspected of mild MAOI properties. May increase the risk of hypertensive crisis.
- 5-MeO-xxT - Suspected of mild MAOI properties. May increase the risk of hypertensive crisis.
- DOx
- aMT - aMT has MAOI properties which may interact unfavorably with amphetamines.
- MAOIs - MAO-B inhibitors can increase the potency and duration of phenethylamines unpredictably. MAO-A inhibitors with amphetamine can lead to hypertensive crises.
Reagent results
Exposing compounds to the reagents gives a colour change which is indicative of the compound under test.
| Marquis | Mecke | Mandelin | Liebermann | Froehde | Robadope |
|---|---|---|---|---|---|
| Orange - red | No reaction | slow (dark) green | Orange - red | No reaction | Pink |
| Ehrlich | Hofmann | Simon’s | Scott | Folin | |
| No reaction | No reaction | No reaction | No reaction | Light orange | |
Legal status
Internationally, amphetamine (and its isomers dextroamphetamine and levoamphetamine) are Schedule II controlled substances under the United Nations 1971 Convention on Psychotropic Substances.[56]
- Australia: Amphetamine is a Schedule 8 controlled substance.[57] Personal quantities under 1.5 grams are decriminalized in the Australian Capital Territory (ACT) as of 28 October 2023.[58]
- Austria: Amphetamine is illegal to possess, produce and sell under the SMG (Suchtmittelgesetz Österreich).[59]
- Brazil: Amphetamine is a Class A3 psychoactive substance.[60]
- Canada: Amphetamine is a Schedule I drug in Canada.[61]
- Finland: Amphetamine is a prohibited substance, and it's illegal to possess, buy, sell or manufacture under the Finnish Narcotics Act.[62]
- France: Amphetamine is scheduled as a "stupéfiant", i.e. a recognized drug of abuse. It is illegal to possess, buy, sell or manufacture, and is not prescriptible.[63]
- Germany: Amphetamine was added to the Opiumgesetz (Opium Act) in 1941.[64] In accordance to the Narcotics Act reform of 1981, it is controlled under Anlage III BtMG (Narcotics Act, Schedule III).[65] It can only be prescribed on a narcotic prescription form.
- Japan: Amphetamine is prohibited even for medical use in Japan.[66]
- Luxembourg: Amphetamine is a prohibited substance for recreational use. [67]
- The Netherlands: Amphetamine is a List I controlled substance.[68]
- New Zealand: Amphetamine is a Class B controlled substance.[69]
- Poland: Amphetamine is a Group II-P controlled substance.[70]
- South Korea: Amphetamine is prohibited even for medical use in South Korea in compliance with the United Nations Convention on Psychotropic Substances.[71]
- Sweden: Amphetamine is classified as a drug by the United Nations and is included in list P II in the 1971 Psychotropic Convention, as well as in list II in Sweden.[72]
- Switzerland: Amphetamine is a controlled substance specifically named under Verzeichnis A. Medicinal use is permitted.[73]
- Thailand: Amphetamine is classified as a category 1 narcotic under the Thai Narcotic Act of 2012.[74]
- United Kingdom: Amphetamine is a Class B drug in the United Kingdom.[75]
- United States: Amphetamine is a Schedule II controlled substance in the United States.[76]
See also
External links
- Amphetamine (Wikipedia)
- Amphetamine (Erowid Vault)
- Amphetamine (Isomer Design)
- Amphetamine (DrugBank)
- Amphetamine (Drugs.com)
Literature
- Galli, A., Poulsen, N.W., Sulzer, D., & Sonders, M.S. (2005). Mechanisms of neurotransmitter release by amphetamines: a review. Progress in Neurobiology, 75 6, 406-33. https://doi.org/10.1016/j.pneurobio.2005.04.003
- Berman, S. M., Kuczenski, R., McCracken, J. T., & London, E. D. (2009). Potential adverse effects of amphetamine treatment on brain and behavior: a review. Molecular Psychiatry, 14(2), 123. https://doi.org/10.1038/mp.2008.90.
- Baumann, M., Carroll, F.I., Dersch, C.M., Partilla, J.S., Rothman, R.B., Romero, D., & Rice, K. (2001). Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse, 39 1, 32-41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3
References
- ↑ "Drugbank - Amphetamine
- ↑ Kish, S. J. (17 June 2008). "Pharmacologic mechanisms of crystal meth". Canadian Medical Association Journal. 178 (13): 1679–1682. doi:10.1503/cmaj.071675. ISSN 0820-3946.
- ↑ 3.0 3.1 Heal, D. J., Smith, S. L., Gosden, J., Nutt, D. J. (June 2013). "Amphetamine, past and present – a pharmacological and clinical perspective". Journal of Psychopharmacology. 27 (6): 479–496. doi:10.1177/0269881113482532. ISSN 0269-8811.
- ↑ 4.0 4.1 Rasmussen, N. (21 February 2006). "Making the First Anti-Depressant: Amphetamine in American Medicine, 1929-1950". Journal of the History of Medicine and Allied Sciences. 61 (3): 288–323. doi:10.1093/jhmas/jrj039. ISSN 0022-5045.
- ↑ Angrist, B., Sudilovsky, A. (1978). "Stimulants". In Iversen, L. L., Iversen, S. D., Snyder, S. H. Central Nervous System Stimulants: Historical Aspects and Clinical Effects. Handbook of Psychopharmacology. Springer US. pp. 99–165. doi:10.1007/978-1-4757-0510-2_3. ISBN 9781475705102.
- ↑ United Nations Treaty Collection
- ↑ Hodgkins, P., Shaw, M., McCarthy, S., Sallee, F. R. (1 March 2012). "The pharmacology and clinical outcomes of amphetamines to treat ADHD: does composition matter?". CNS drugs. 26 (3): 245–268. doi:10.2165/11599630-000000000-00000. ISSN 1179-1934.
- ↑ Billiard, M. (June 2008). "Narcolepsy: current treatment options and future approaches". Neuropsychiatric Disease and Treatment. 4 (3): 557–566. ISSN 1176-6328.
- ↑ Sobel, LJ., Bansal, R., Maia, TV., Sanchez, J., Mazzone, L., Durkin, K., Liu, J., Hao, X., Ivanov, I., Miller, A., Greenhill, LL., Peterson, BS. (1 August 2010). "Basal Ganglia Surface Morphology and the Effects of Stimulant Medications in Youth with Attention-Deficit/Hyperactivity Disorder". The American Journal of Psychiatry. 167 (8): 977–986. doi:10.1176/appi.ajp.2010.09091259.
- ↑ Frodl, T., Skokauskas, N. (February 2012). "Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects". Acta Psychiatrica Scandinavica. 125 (2): 114–26. doi:10.1111/j.1600-0447.2011.01786.x. PMID 22118249.
- ↑ Westover, A. N., McBride, S., Haley, R. W. (1 April 2007). "Stroke in Young Adults Who Abuse Amphetamines or Cocaine: A Population-Based Study of Hospitalized Patients". Archives of General Psychiatry. 64 (4): 495. doi:10.1001/archpsyc.64.4.495. ISSN 0003-990X.
- ↑ Edeleano, L. (January 1887). "Ueber einige Derivate der Phenylmethacrylsäure und der Phenylisobuttersäure". Berichte der deutschen chemischen Gesellschaft. 20 (1): 616–622. doi:10.1002/cber.188702001142. ISSN 0365-9496.
- ↑ Sulzer, D., Sonders, M. S., Poulsen, N. W., Galli, A. (April 2005). "Mechanisms of neurotransmitter release by amphetamines: A review". Progress in Neurobiology. 75 (6): 406–433. doi:10.1016/j.pneurobio.2005.04.003. ISSN 0301-0082.
- ↑ Bett, W. R. (1 August 1946). "Benzedrine Sulphate in Clinical Medicine". Postgraduate Medical Journal. 22 (250): 205–218. doi:10.1136/pgmj.22.250.205. ISSN 0032-5473.
- ↑ Rasmussen, N. (September 2011). "Medical Science and the Military: The Allies' Use of Amphetamine during World War II". The Journal of Interdisciplinary History. 42 (2): 205–233. doi:10.1162/JINH_a_00212. ISSN 0022-1953.
- ↑ Defalque, R. J., Wright, A. J. (April 2011). "Methamphetamine for Hitler's Germany: 1937 to 1945". Bulletin of Anesthesia History. 29 (2): 21–32. doi:10.1016/S1522-8649(11)50016-2. ISSN 1522-8649.
- ↑ "Historical overview of methamphetamine". Vermont Department of Health. Government of Vermont. Archived from the original on 5 October 2012. Retrieved 29 January 2012.
- ↑ 18.0 18.1 18.2 18.3 Mohan, J. (2014), World Drug Report 2014, United Nations Office on Drugs and Crime, retrieved 18 August 2014
- ↑ PubChem - Amphetamine, National Center for Biotechnology Information, retrieved 13 October 2013
- ↑ 20.0 20.1 Nestler, E. J., Hyman, S. E., Malenka, R. C. (2009). Molecular neuropharmacology: a foundation for clinical neuroscience (2nd ed ed.). McGraw-Hill Medical. ISBN 9780071481274.
- ↑ Miller, G. M. (January 2011). "The Emerging Role of Trace Amine Associated Receptor 1 in the Functional Regulation of Monoamine Transporters and Dopaminergic Activity". Journal of neurochemistry. 116 (2): 164–176. doi:10.1111/j.1471-4159.2010.07109.x. ISSN 0022-3042.
- ↑ Drugbank - Amphetamine targets
- ↑ TA1 receptor | http://www.iuphar-db.org/DATABASE/ObjectDisplayForward?objectId=364
- ↑ Lewin, A. H., Miller, G. M., Gilmour, B. (December 2011). "Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class". Bioorganic & Medicinal Chemistry. 19 (23): 7044–7048. doi:10.1016/j.bmc.2011.10.007. ISSN 0968-0896.
- ↑ Goodman, L. S., Brunton, L. L., Chabner, B., Knollmann, B. C., eds. (2011). Goodman & Gilman’s pharmacological basis of therapeutics (12th ed ed.). McGraw-Hill. ISBN 9780071624428.
- ↑ Eiden, L. E., Weihe, E. (January 2011). "VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse: VMAT2 and addiction". Annals of the New York Academy of Sciences. 1216 (1): 86–98. doi:10.1111/j.1749-6632.2010.05906.x. ISSN 0077-8923.
- ↑ 27.0 27.1 DailyMed - ADDERALL XR- dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine sulfate and amphetamine aspartate capsule, extended release
- ↑ 28.0 28.1 28.2 Sinha, A., Lewis, O., Kumar, R., Yeruva, S. L. H., Curry, B. H. (2016). "Adult ADHD Medications and Their Cardiovascular Implications". Case Reports in Cardiology. 2016: 2343691. doi:10.1155/2016/2343691. ISSN 2090-6404.
- ↑ 29.0 29.1 Dolder, P. C., Strajhar, P., Vizeli, P., Hammann, F., Odermatt, A., Liechti, M. E. (7 September 2017). "Pharmacokinetics and Pharmacodynamics of Lisdexamfetamine Compared with D-Amphetamine in Healthy Subjects". Frontiers in Pharmacology. 8: 617. doi:10.3389/fphar.2017.00617. ISSN 1663-9812.
- ↑ Poulton, A. S., Hibbert, E. J., Champion, B. L., Nanan, R. K. H. (25 April 2016). "Stimulants for the Control of Hedonic Appetite". Frontiers in Pharmacology. 7: 105. doi:10.3389/fphar.2016.00105. ISSN 1663-9812.
- ↑ Nelson, P. E., Moffat, A. C. "Amphetamines and Related Stimulants: Chemical, Biological, Clinical, and Sociological Aspects". Detection and Identification of Amphetamine and Related Stimulants.
- ↑ Biederman, J., Spencer, T. J., Wilens, T. E., Weisler, R. H., Read, S. C., Tulloch, S. J. (December 2005). "Long-term safety and effectiveness of mixed amphetamine salts extended release in adults with ADHD". CNS spectrums. 10 (12 Suppl 20): 16–25. doi:10.1017/s1092852900002406. ISSN 1092-8529.
- ↑ Pigeau, R, Naitoh, P, Buguet, A, McCann, C, Baranski, J, Taylor, M, Thompson, M, MacK, I (December 1995). "Modafinil, d-amphetamine and placebo during 64 hours of sustained mental work. I. Effects on mood, fatigue, cognitive performance and body temperature". Journal of Sleep Research. 4 (4): 212–228. doi:10.1111/j.1365-2869.1995.tb00172.x. ISSN 1365-2869.
- ↑ Broadley, K. J. (1 March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. ISSN 0163-7258.
- ↑ Nutt DJ, King LA, Phillips LD (November 2010). "Drug harms in the UK: a multicriteria decision analysis". Lancet. 376 (9752): 1558–1565. CiteSeerX 10.1.1.690.1283
 . doi:10.1016/S0140-6736(10)61462-6. PMID 21036393. Unknown parameter
. doi:10.1016/S0140-6736(10)61462-6. PMID 21036393. Unknown parameter |s2cid=ignored (help) - ↑ Nutt, D., King, L. A., Saulsbury, W., Blakemore, C. (24 March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". The Lancet. 369 (9566): 1047–1053. doi:10.1016/S0140-6736(07)60464-4. ISSN 0140-6736.
- ↑ Human health effects - Amphetamine | http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@rn+@rel+300-62-9
- ↑ Carvalho, M., Carmo, H., Costa, V. M., Capela, J. P., Pontes, H., Remião, F., Carvalho, F., Bastos, M. de L. (1 August 2012). "Toxicity of amphetamines: an update". Archives of Toxicology. 86 (8): 1167–1231. doi:10.1007/s00204-012-0815-5. ISSN 1432-0738.
- ↑ Miyazaki, I., Asanuma, M. (June 2008). "Dopaminergic neuron-specific oxidative stress caused by dopamine itself". Acta Medica Okayama. 62 (3): 141–150. doi:10.18926/AMO/30942. ISSN 0386-300X.
- ↑ Advokat, C. (July 2007). "Literature Review: Update on Amphetamine Neurotoxicity and Its Relevance to the Treatment of ADHD". Journal of Attention Disorders. 11 (1): 8–16. doi:10.1177/1087054706295605. ISSN 1087-0547.
- ↑ Bowyer, J. F., Hanig, J. P. (14 November 2014). "Amphetamine- and methamphetamine-induced hyperthermia: Implications of the effects produced in brain vasculature and peripheral organs to forebrain neurotoxicity". Temperature: Multidisciplinary Biomedical Journal. 1 (3): 172–182. doi:10.4161/23328940.2014.982049. ISSN 2332-8940.
- ↑ Chetsawang, J., Mukda, S., Srimokra, R., Govitrapong, P., Chetsawang, B. (3 July 2017). "Role of Melatonin in Reducing Amphetamine-Induced Degeneration in Substantia Nigra of Rats via Calpain and Calpastatin Interaction". Journal of Experimental Neuroscience. 11: 1179069517719237. doi:10.1177/1179069517719237. ISSN 1179-0695.
- ↑ Leeboonngam, T., Pramong, R., Sae-Ung, K., Govitrapong, P., Phansuwan-Pujito, P. (April 2018). "Neuroprotective effects of melatonin on amphetamine-induced dopaminergic fiber degeneration in the hippocampus of postnatal rats". Journal of Pineal Research. 64 (3). doi:10.1111/jpi.12456. ISSN 1600-079X.
- ↑ Amphetamine - human health effects | http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+3287
- ↑ Amphetamines: Drug Use and Abuse: Merck Manual Home Edition, 2007
- ↑ Pérez-Mañá, C., Castells, X., Torrens, M., Capellà, D., Farre, M. (2 September 2013). Cochrane Drugs and Alcohol Group, ed. "Efficacy of psychostimulant drugs for amphetamine abuse or dependence". Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD009695.pub2. ISSN 1465-1858.
- ↑ "Adderall XR Prescribing Information" | http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf
- ↑ Stolerman, I. P., ed. (2010). Encyclopedia of psychopharmacology. Springer. ISBN 9783540687092.
- ↑ "Miscellaneous Sympathomimetic Agonists" | http://accessmedicine.mhmedical.com/content.aspx?bookid=374§ionid=41266218&jumpsectionID=41268855
- ↑ 50.0 50.1 50.2 Shoptaw, S. J., Kao, U., Ling, W. (21 January 2009). Cochrane Drugs and Alcohol Group, ed. "Treatment for amphetamine psychosis". Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD003026.pub3. ISSN 1465-1858.
- ↑ Hofmann, F. G. (1983). A handbook on drug and alcohol abuse: the biomedical aspects (2nd ed ed.). Oxford University Press. ISBN 9780195030563.
- ↑ http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf
- ↑ Greenwald, M. K., Lundahl, L. H., Steinmiller, C. L. (December 2010). "Sustained Release d-Amphetamine Reduces Cocaine but not 'Speedball'-Seeking in Buprenorphine-Maintained Volunteers: A Test of Dual-Agonist Pharmacotherapy for Cocaine/Heroin Polydrug Abusers". Neuropsychopharmacology. 35 (13): 2624–2637. doi:10.1038/npp.2010.175. ISSN 0893-133X.
- ↑ Siciliano, C. A., Saha, K., Calipari, E. S., Fordahl, S. C., Chen, R., Khoshbouei, H., Jones, S. R. (10 January 2018). "Amphetamine Reverses Escalated Cocaine Intake via Restoration of Dopamine Transporter Conformation". The Journal of Neuroscience. 38 (2): 484–497. doi:10.1523/JNEUROSCI.2604-17.2017. ISSN 0270-6474.
- ↑ Krystal, J. H., Perry, E. B., Gueorguieva, R., Belger, A., Madonick, S. H., Abi-Dargham, A., Cooper, T. B., MacDougall, L., Abi-Saab, W., D’Souza, D. C. (1 September 2005). "Comparative and Interactive Human Psychopharmacologic Effects of Ketamine and Amphetamine: Implications for Glutamatergic and Dopaminergic Model Psychoses and Cognitive Function". Archives of General Psychiatry. 62 (9): 985. doi:10.1001/archpsyc.62.9.985. ISSN 0003-990X.
- ↑ "CONVENTION ON PSYCHOTROPIC SUBSTANCES 1971" (PDF). United Nations. Retrieved December 19, 2019.
- ↑ "POISONS STANDARD DECEMBER 2019". Office of Parliamentary Counsel. Retrieved December 19, 2019.
- ↑ https://www.health.act.gov.au/about-our-health-system/population-health/drug-law-reform
- ↑ https://www.ris.bka.gv.at/GeltendeFassung.wxe?Abfrage=Bundesnormen&Gesetzesnummer=10011053
- ↑ https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-784-de-31-de-marco-de-2023-474904992
- ↑ Controlled Drugs and Substances Act | http://laws-lois.justice.gc.ca/eng/acts/C-38.8/page-24.html#h-28
- ↑ Valtioneuvoston asetus huumausaineina pidettävistä aineista, valmisteista ja kasveista
- ↑ Arrêté du 22 février 1990 fixant la liste des substances classées comme stupéfiants
- ↑ "Sechste Verordnung über die Unterstellung weiterer Stoffe unter die Bestimmungen des Opiumgesetzes" (in German). Reichsministerium des Innern. Retrieved December 25, 2019.
- ↑ "Anlage III BtMG" (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 19, 2019.
- ↑ UNODC - Bulletin on Narcotics - 1957 Issue 3 - 002
- ↑ Règlement grand-ducal du 20 mars 1974 concernant certaines substances psychotropes
- ↑ "Opiumwet" (in Dutch). Ministerie van Binnenlandse Zaken en Koninkrijksrelaties. Retrieved December 19, 2019.
- ↑ "Schedule 2 - Class B controlled drugs". Parliamentary Counsel Office. Retrieved December 19, 2019.
- ↑ Ustawa z dnia 24 kwietnia 2015 r. o zmianie ustawy o przeciwdziałaniu narkomanii oraz niektórych innych ustaw (Dz.U. z 2015 r. poz. 875).
- ↑ https://web.archive.org/web/20160331074842/https://treaties.un.org/pages/ViewDetails.aspx?src=TREATY&mtdsg_no=VI-16&chapter=6&lang=en
- ↑ Läkemedelsverkets föreskrifter (LVFS 1997:12) om förteckningar över narkotika, konsoliderad version till och med LVFS 2010:1
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
- ↑ Thai Narcotic Act of 2012 | http://narcotic.fda.moph.go.th/faq/upload/Thai%20Narcotic%20Act%202012.doc._37ef.pdf
- ↑ Misuse of Drugs Act 1971
- ↑ Controlled Drugs and Substances Act | http://www.fda.gov/regulatoryinformation/legislation/ucm148726.htm