Ketamine
| Summary sheet: Ketamine |
| Ketamine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common names | Ketamine, K, Ket, Kitty, Special K, Cat Tranquilizer, Ketaset, Ketalar, Ketanest, Vitamin K, Purple, Jet | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substitutive name | Ketamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Systematic name | (RS)-2-(2-Chlorophenyl)-2-(methylamino)cyclohexanone | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Psychoactive class | Dissociative | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical class | Arylcyclohexylamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Amphetamines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cocaine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Benzodiazepines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MAOIs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Trazodone | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Grapefruit | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alcohol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GHB | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GBL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Opioids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tramadol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ketamine (also known as 2-Cl-2’-oxo-PCM, Ket, K, Special K, vitamin K,[5] Kitty, 2-CXM, and others) is a classical dissociative substance of the arylcyclohexylamine class. It is perhaps the best-known and archetypal member of the dissociatives, a diverse group which includes PCP, methoxetamine, DXM, and nitrous oxide. The mechanism of action is not fully known, although blocking of the NMDA glutamate receptor is thought to be involved.
Developed in 1963 by Parke-Davis Laboratories, it was originally intended as a replacement for the surgical anesthetic phencyclidine (PCP).[6] It is now widely used in human and veterinary medicine, typically in surgical and intensive care settings. Recently, it has received significant clinical research following the discovery that it can rapidly relieve treatment-resistant depression and suicidal ideation.[7]
Recreational use was first reported amongst medicinal chemists in the United States in 1967,[8] and became more widespread in Europe in the 1990s, where it gained popularity as an adulterant in ecstasy tablets.[9][10] Today, it is associated in popular culture with the nightclub and rave scenes.[11]
Subjective effects include motor control loss, pain relief, internal hallucinations, memory suppression, conceptual thinking, immersion enhancement, euphoria, and depersonalization / dissociation. The effects are similar to PCP and DXM but with a shorter duration and a rapid onset. It is known for producing relatively "pure" dissociation, without as much stimulation and mania as PCP or MXE.
Additionally, the effects of ketamine are highly dose-dependent.[12] At lower doses, users report alcohol-like disinhibition and relaxation effects. At higher doses, however, it reportedly produces a hallucinogenic trance-like state (called a "k-hole") that is often described as an "out-of-body" or "near-death" experience.
It has moderate to high abuse potential. Chronic use (i.e. high dose, repeat administration) is linked with compulsive redosing and psychological dependence. Additionally, the health risks of chronic or heavy use are not well-studied; however, there is increasing evidence that it can cause bladder dysfunction[13] and some evidence of cognitive and memory issues (see this section for more information).[14][15]
It is highly advised to use harm reduction practices if using this substance.
History and culture
Ketamine was first synthesized at Parke Davis Laboratories by the American scientist Calvin Stevens. Stevens was searching for a new anesthetic to replace PCP, which was deemed not suitable for use in humans because of the severe hallucinogenic effects it produced upon recovery of consciousness.[16]
Upon being patented in Belgium in 1963, ketamine was initially used as a veterinary anesthetic. After being patented by Parke-Davis for human and animal use in 1966, it became available by prescription in 1969 in the form of ketamine hydrochloride, under the name of Ketalar.[citation needed]
The United States Food and Drug Administration approved it for human consumption in 1970. Due to its favorable sympathomimetic properties and its wide margin of safety, it was administered as a field anaesthetic to soldiers during the Vietnam War.[17]
Ketamine is on the World Health Organization’s “Essential Drugs List”, a list of the safest and most effective drugs needed in a modern health system.[18]
Common names
Street names include "Special K", "K", "Kit Kat", "kitty", and "horse/dog/cat tranquilizer" (which refers to its use in veterinary medicine), "Cat Valium", and "Jet".[19]
Chemistry
Ketamine, or (RS)-2-(2-Chlorophenyl)-2-(methylamino)cyclohexanone, belongs to a class of synthetic organic compounds called arylcyclohexylamines. Arylcyclohexylamines are named for their chemical structures, which include a cyclohexane ring bound to an aromatic ring along with an amine group.
The chemical structure contains a phenyl ring with a chlorine substituent at R2 bonded to a cyclohexane ring substituted with an -Oxo group (cyclohexanone). An amino methyl chain (-N-CH3) is bound to the same location (R1) of the cyclohexanone ring.
Enantiomers
Ketamine is a mixture of equal amounts of two enantiomers: esketamine and arketamine.
Esketamine is a more potent NMDA receptor antagonist and dissociative hallucinogen than arketamine.[20] Because of the hypothesis that NMDA receptor antagonism underlies the antidepressant effects of ketamine, esketamine was developed as an antidepressant.
However, multiple other NMDA receptor antagonists, including memantine, lanicemine, rislenemdaz, rapastinel, and 4-chlorokynurenine, have thus far failed to demonstrate sufficient effectiveness for depression.[20]
Furthermore, animal research indicates that arketamine, the enantiomer with a weaker NMDA receptor antagonism, as well as (2R,6R)-hydroxynorketamine, the metabolite with negligible affinity for the NMDA receptor but a potent alpha-7 nicotinic receptor antagonist may have antidepressive action.[20]
Pharmacology
Ketamine is classified as a non-competitive NMDA receptor antagonist.
The NMDA receptor, an ionotropic glutamate receptor, allows for electrical signals to pass between neurons in the brain and spinal column; for the signals to pass, the receptor must be open. Dissociatives close the NMDA receptors by blocking them. This disconnection of neurons leads to loss of feeling, difficulty moving, and eventually, the state known as the “K-hole”.
It is one of the most recognizable dissociatives, a diverse group that includes phencyclidine (PCP), methoxetamine (MXE), dextromethorphan (DXM), and nitrous oxide.
At high, fully-anesthetic level doses, ketamine has also been found to bind to μ-opioid receptors type 2 in cultured human neuroblastoma cells without agonist activity[21] and to sigma receptors in rats.[22] Also, ketamine interacts with muscarinic receptors, descending monoaminergic pain pathways and voltage-gated calcium channels.[23]
At subanesthetic and fully anesthetic doses, ketamine has been found to block serotonin depletion in the brain by inhibiting 5-HT receptors rather than through monoamine oxidase inhibition.[24]
The estimated bioavailabilities are as follows: nasal (45%), oral (17%), rectal (25%).[25]
Research
Psychoplastogen
Ketamine is a psychoplastogen,[26] which refers to a compound capable of promoting rapid and sustained neuroplasticity.
Subjective effects
At higher doses, ketamine is reported to produce significant cognitive impairment and alteration, often resulting in the complete loss of symbolic reasoning, communication, and fine motor abilities. These changes appear to coincide with its transpersonal and therapeutic effects.
Some users also report an antidepressive afterglow, which may last days or weeks.
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Sedation - Ketamine is reported to have a moderate sedating effect. It discourages physical activity and can render the user immobile at higher doses.
- Spontaneous bodily sensations - The ketamine "body high" can be described as a sharp, pleasurable, tingling sensation that is location-specific to the hands, feet, and head. This manifests as an out-of-body sensation or a general disconnection between the mind and body, which can be accompanied by states of physical euphoria.
- Changes in felt bodily form & Changes in felt gravity - Ketamine can strongly alter the way the user perceives their bodily form in a dose-dependent manner. At k-hole doses, the user may feel as if they are entirely disconnected from their body and the earth.
- Physical euphoria - Physical euphoria may be present in some users. However, the effect occurs far less strongly and reliably than with opiates or MDMA.
- Perception of bodily lightness - The user may feel as if the body is floating and has become entirely weightless. At low doses, this effect is reported to be oddly stimulating, promoting physical activity by making the body feel effortless to move.
- Motor control loss - A loss of gross and fine motor control alongside balance and coordination is common and becomes pronounced at higher doses. Users are advised to be sitting during the onset in case they fall over and injure themselves.
- Increased blood pressure[27] - Shown to occur at higher doses.
- Dehydration - Minor to moderate. More of a risk in hot, high-activity settings such as dance floors.
- Tactile suppression - The sense of touch may be entirely suppressed, creating feelings of numbness within the extremities.
- Pain relief - Very prominent. Most physical sensations are suppressed on ketamine.
- Optical sliding - The user's eyes may wiggle and shake (a condition known as nystagmus), particularly at higher doses. This effect is temporary and usually not a cause for concern unless it persists after other effects have worn off.
- Dizziness - Some users report dizziness while under the influence of ketamine.
- Increased salivation
- Nausea - Uncommon, but more likely at higher doses and near the peak of the experience.
- Gustatory hallucination
- Difficulty urinating
- Decreased libido - Unlike stimulants and many other substances, ketamine tends to strongly decreases libido, making sexual activity unappealing and difficult to perform; coincides with tactile suppression and orgasm suppression.
- Orgasm suppression - Strongly inhibits orgasms and the normal sexual arousal response at moderate to high doses.
- Physical autonomy - Relatively uncommon, but may occur in some users. Users may feel as if their body is performing gestures and movements outside of their own control. However, these disturbances tend to be simple and short-lived.
- Pupil dilation[citation needed]
Visual effects 
-
The visual effects of ketamine are highly suppressive and distortive. This is one of the many reasons one should never drive or operate machinery while under the influence of ketamine!
Suppression
- Double vision - Prevalent at moderate to heavy doses and can make reading text impossible unless closing one eye.
- Pattern recognition suppression - Generally occurs at higher doses and makes one unable to recognize and interpret perceivable visual data.
- Acuity suppression
- Frame rate suppression - Dose-dependent and prominent at higher doses.
Distortions
Geometry
The visual geometry produced by ketamine can be described as very brightly colored in scheme when compared to that of other, less visually disconnecting dissociatives like MXE and PCP, but not as complex as the geometry evoked by DXM or any psychedelic.
It does not extend beyond level 4 and can be comprehensively described in its variations as: simplistic in complexity, algorithmic in style, synthetic in feel, unstructured in organization, dimly lit in lighting, multicoloured in scheme, glossy in shading, soft in edges, large in size, fast in speed, smooth in motion, equal in rounded and angular corners, immersive in its depth and consistent in its intensity.
Beyond simple geometric shape hallucinations, high doses of ketamine that enter the "hole" can yield a white glow and other low complexity hallucinations like ghosts of people formed of white strings.
Hallucinatory states
Higher doses of ketamine can produce a full range of high-level hallucinatory states in a fashion that is less consistent and reproducible than that of many common psychedelics. These effects include:
- Machinescapes
- Internal hallucination (autonomous entities; settings, sceneries, and landscapes; perspective hallucinations and scenarios and plots) - This effect can be comprehensively described through its variations as delirious in believability, fixed in style, equal in new experiences and memory replays in content, autonomous in controllability and solid in style.
- External hallucination (autonomous entities; settings, sceneries, and landscapes; perspective hallucinations and scenarios and plots) - This effect can be comprehensively described through its variations as delirious in believability, autonomous in controllability and solid in style. The most common theme for this effect to follow is one of experiencing and talking to friends when they are not actually present.
Cognitive effects 
-
- Analysis suppression - Users report that it is difficult to think normally or logically while under the influence of ketamine. Normal cognition and working memory are impaired in a dose-dependent manner. However, the trade-off is that creative or non-linear thinking faculties may become enhanced (see conceptual thinking).
- Anxiety suppression - Anxiety suppression is notable at all doses but not as selective as the effect of benzodiazepines or other GABAergics.
- Cognitive euphoria - Users report moderate to strong states of cognitive euphoria which tend to occur during the come-up phase; however, appears to be less pronounced compared to stimulants, entactogens, and opioids.
- Compulsive redosing - Due to its euphoric effects, rapid onset, and short duration, ketamine can cause compulsive re-dosing in some individuals. It is strongly advised to employ strategies to limit intake and prevent abuse.
- Conceptual thinking - Ketamine produces conceptual or non-linear thinking in a manner that can stimulate one's artistic or creative faculties. Users commonly report entering dream-like, highly complex, and abstract mental spaces that frees them from the boundaries of normal cognition and promotes new insight into their lives and mental patterns.
- Déjà vu - The user may experience a powerful sensation of déjà vu. This effect is uncommon but less-so than with other substances.
- Delusion - Delusions appear to occur more commonly on ketamine than other substances (e.g. stimulants, psychedelics). This effect often coincides with its ego inflation component. It is strongly advised to avoid ketamine if you are susceptible to mental disorders like schizophrenia or bipolar disorder as it may exacerbate delusions and trigger psychosis.
- Depersonalization & Derealization
- Depression reduction
- Disinhibition - Low doses produce disinhibition in a similar manner as alcohol. As a result, it is sometimes used for this purpose in parties and raves. Higher doses will result in the opposite effect: social withdrawal and inability to communicate normally.
- Dream potentiation
- Ego inflation - Lower doses may result in ego inflation similar to that observed on alcohol, benzodiazepines, or cocaine. Can also occur with k-hole doses, manifesting as delusions of grandeur.
- Focus suppression - The ability to focus on a single task or object may be strongly suppressed. Its mental effect can be described as "scattering" and "non-linear"; occurs alongside analysis suppression.
- Immersion enhancement - The user's sense of immersion may be significantly enhanced, particularly when viewing visual media. It is considered to be one of the most immersion-enhancing substances known.
- Increased music appreciation - Ketamine may increase one's appreciation of music depending on dose and setting. However, this effect is generally less consistent than the effect of psychedelics or entactogens. Sometimes the opposite effect occurs, causing music to sound alien and unpleasant.
- Introspection - Some user reports suggest ketamine may enhance introspection; however, this effect appears to be much less consistent and robust than psychedelics and entactogens. It should be noted that there is limited evidence showing ketamine has psychotherapeutic benefits.
- Memory suppression - Strongly suppresses short and long-term memory (in a dose-dependent manner) for the duration of the experience. Heavy doses appear to be able to temporarily turn off one's memory altogether and produce amnesia.
- Personal bias suppression - Some user reports suggest ketamine may suppress one's personal bias; however, this effect appears to be much less consistent and robust than the effect of psychedelics or entactogens.
- Mania
- Psychosis - Psychosis appear to be more common on ketamine than other substances (e.g. stimulants, psychedelics) and appears to coincide with the delusion effect. It is strongly advised to avoid ketamine if you are susceptible to mental disorders like schizophrenia or bipolar disorder as it may exacerbate delusions and trigger psychosis.
- Spatial disorientation - Spatial orientation is very prominent and occurs in a dose-dependent manner. As a result, the user should carefully analyze any environment in which they are taking ketamine to avoid becoming lost or injuring themselves.
- Suggestibility enhancement - The user may be rendered significantly more suggestible during and after ketamine administration. This may be a result of ketamine's effect on cognition and susceptibility to delusions and psychosis.
- Thought deceleration - Thoughts may be perceived by the user as if they are stiff, frozen, or in slow-motion. Dissociatives are known to produce this effect more strongly than other substances.
- Time distortion - Ketamine prominently alters the subjective experience of time, particularly at the k-hole threshold. In this state, the user is rendered unable to tell how much time has passed; some users report that it can feel like an entire lifetime in the span of half an hour. Others report being transported to a dimension seemingly beyond time and space until the effects wear off.
- Addiction suppression - Ketamine is actively studied as a possible treatment for alcohol use disorder with promising early results.[28][29]
Auditory effects 
Disconnective effects 
-
Ketamine is widely known for its disconnective effects, which together can be referred to as "dissociation". The following lists a breakdown of the different forms of sensory and mental disconnection reported on ketamine.
- Cognitive disconnection
- Physical disconnection
- Visual disconnection - This eventually results in the experience of the notorious "k-hole" or, more specifically, holes, spaces and voids alongside of structures.
Multi-sensory effects 
-
- Synaesthesia - Synaesthesia is sometimes reported on ketamine, especially at higher doses. Some users have reported the experience of being able to "hear colors" or "see sounds" in the k-hole state. However, it is not clear if ketamine is capable of producing synaesthesia as a normal effect or if it is merely eliciting it in predisposed individuals. More research is needed to understand the etiology of this reported effect.
Transpersonal effects 
-
Transpersonal effects are sometimes reported on ketamine and other dissociatives. However, these effects appear to occur with far less consistency and robustness than those observed on psychedelics and entactogens.
It should be noted that there is currently limited clinical evidence that ketamine has psychotherapeutic benefits and some evidence that it promotes disordered thinking.[citation needed]
Experience reports
Anecdotal reports which describe the effects of this compound within our experience index include:
- Experience: 500 mg Ketamine (Insufflated) - A Nauseating Voyage Through Parallel Universes
- Experience:150 mg ketamine
- Experience:260 mg Ketamine (insufflated) - Lost in Paisley
- Experience:300mg Ketamine (Insufflated) - The Void: Finding peace in death
- Experience:50mg Ketamine (esketamine) i.m. - Exploring space heights and ocean depths
- Experience:75mg Ketamine (insufflated) - Wandering through the winter night
- Experience:Ketamine (200mg nasal) Quasi sexual experience
- Experience:Ketamine (80mg, smoked) - Untitled
- Experience:Ketamine (unknown dosage) - States of unity and interconnectedness
Additional experience reports can be found here:
Research
Near-death experience
Most people who were able to remember their dreams during ketamine anesthesia report near-death experiences (NDE) when the widest possible definition of a NDE is used.[31] Ketamine can reproduce features which have commonly been associated with NDE's.(Domino et al., 1965; Rumpf ,1969; Collier, 1972; Siegel,1978, 1980,1981; Stafford, 1977; Lilly, 1978; Grinspoon and Bakalar, 1981; White, 1982; Ghoniem et al., 1985; Sputz, 1989; Jansen, 1989a,b, 1990b, 1993, 1995, 1996). A 2019 large-scale study found that most drug-induced NDEs were linked to ketamine.[30] Ketamine can be used to study the death (thanatology), and to assist death-rebirth psychotherapy.[32]
Novel antidepressant
Ketamine has been shown to be effective for patients suffering from chronic depression and bipolar disorder. Studies have shown[33][34] that the effect of the drug is immediate or within 2 hours and consistent in relieving a patient’s depressive and/or suicidal symptoms, lasting up to 3 days after a single dose. In comparison, common antidepressants such as fluoxetine (Prozac) can take weeks to show therapeutic effects.
Ketamine is a racemate composed of the R-(−)-ketamine enantiomer (arketamine) and the S-(+)-ketamine enantiomer (esketamine). Esketamine inhibits the reuptake of the dopamine transporter about 8-fold more potently than does arketamine, and so is about 8 times more potent as a dopamine reuptake inhibitor.[35] Arketamine appears to be more effective as a rapid-acting antidepressant than esketamine.[36]
A study conducted in mice found that ketamine's antidepressant activity is not caused by ketamine inhibiting NMDAR, but rather by sustained activation of a different glutamate receptor, the AMPA receptor, by a metabolite, (2R,6R)-hydroxynorketamine; as of 2017 it was unknown if this was happening in humans.[37][38] Arketamine is a AMPA receptor agonist.[39]
Anesthetic doses may not produce an antidepressant effect. While the research is limited, between .5 and 1mg/kg IV given over about 40 mins seems to be the optimal dose. [40] A typical anesthetic dose is 1-2 mg/kg given over 2 minutes, followed by .5-1.8mg/kg/hr. Benzodiazepines and GABA agonist (both of which are often used alongside ketamine in anesthesia) may mitigate the antidepressant effect of ketamine.
Janssen Neuroscience, a subsidiary of Johnson & Johnson, market a water-based ketamine nasal spray against depression under the name brand name Spravato. It was was approved as for use by the European Medicines Agency in 2019.[41] The single-use nasal spray devices contain 28 mg of esketamine (32.3 mg esketamine hydrochloride) in 0.2 mL of solution, a concentration of >= 10 - < 20 % (w/w)[42] and delivers its contents in 2 sprays, one per nostril. The devices are meant to be used in conjunction with an oral antidepressant and administered under the supervision of a healthcare provider at a medical center. 1, 2 or 3 devices (i.e. 28 mg, 56 mg or 84 mg of esketamine) may be used per session: if multiple devices are used, patients are instructed to wait for 5 minutes between device to ensure complete uptake of the drug.[43]
Reagent results
Exposing compounds to the reagents gives a colour change which is indicative of the compound under test.
| Marquis | Mecke | Mandelin | Liebermann | Froehde | Gallic | Robadope |
|---|---|---|---|---|---|---|
| No reaction | No reaction | Deep brownish orange/red | Light yellow | No reaction | No reaction | No reaction |
| Ehrlich | Hofmann | Simon’s | Zimmermann | Scott | Folin | |
| No reaction | No reaction | Orange - Pink - Yellow | Slow pink | No reaction | No reaction | |
Toxicity and harm potential
 |
This toxicity and harm potential section is a stub. As a result, it may contain incomplete or even dangerously wrong information! You can help by expanding upon or correcting it. |


Both ketamine and esketamine may increase bladder damage.
Ketamine possesses a strong abuse potential at typical antidepressive doses.[46][47] Ketamine has reported cases of severe bladder and liver injury. Esketamine, a newer nasal spray formulation of Ketamine, does not have any reported cases and is purported to have a better safety profile. However, in recent short-term clinical trials esketamine still more-than-doubled the amount of adverse bladder events when compared to placebo (6-10% vs 1-4%). Although 2/3 of esketamine incidents resolved themselves either without intervention or through a lowering of dosage, any physiological damage is acute and immediate: in typical dose regimens steady-state concentrations are not reached.
Warning: Nasal Administration Insufflation can cause nasal damage, bleeding, and—with repeated long-term use—irreversible damage to the nose and surrounding tissues. Sharing snorting equipment (including banknotes) increases the risk of transmitting blood-borne diseases (such as hepatitis C and HIV). To stay safer: Prepare substances into a fine powder before use, and always use your own clean snorting tool. Limit how much you use in each nostril per session, and rinse your nose with saline within 30–60 minutes after use (after the peak effects) to clear out any leftover material and reduce irritation. If with others, don’t let anyone push you to use. Alternatively: Buccal administration may be used as a harm reduction option. This involves placing the powder (for example, wrapped in a small piece of toilet paper) under the lip, allowing it to absorb through the cheek or gum. This method avoids nasal damage, though it may have different effects and risks, such as irritation to the mouth or gums. Learn more about nasal administration risks. |
It is strongly advised to use harm reduction practices if using this substance.
There is some evidence that ketamine may have antibiotic properties at higher doses.[48] It is unclear how this affects normal human use.
Cognition and well-being
The first large-scale, longitudinal study of ketamine users found that frequent ketamine users (at least 4 days/week, averaging 20 days/month) had increased depression and impaired memory by several measures, including verbal, short-term memory and visual memory. However, infrequent (1–4 days/month, averaging 3.25 days/month) ketamine users and former ketamine users were not found to differ from controls in memory, attention and psychological well-being tests.
This suggests the infrequent use of ketamine does not cause cognitive deficits and that any deficits that might occur may be reversible when ketamine use is discontinued. However, abstinent, frequent, and infrequent users all scored higher than controls on a test of delusional symptoms.[14]
Urinary tract effects
According to a 2010 systematic review, 110 documented reports of irritative urinary tract symptoms from ketamine dependence exist.[49] Urinary tract symptoms have been collectively referred to as "ketamine-induced ulcerative cystitis" or "ketamine-induced vesicopathy" and they include urge incontinence, decreased bladder compliance, decreased bladder volume, and painful haematuria (blood in urine).
The time of onset of lower urinary tract symptoms varies depending, in part, on the severity and chronicity of ketamine use; however, it is unclear whether the severity and chronicity of ketamine use correspond linearly to the presentation of these symptoms. All reported cases where the user consumed greater than 5 grams per day reported symptoms of the lower urinary tract.[50]
A study conducted in 2015 demonstrated that co-administration of EGCG (10mM/kg) in rats receiving a daily dose of ketamine (25 mg/kg/d), significantly reduced ketamine-induced bladder damage to almost control levels, showing potential benefits of EGCG consumption to prevent or reverse ketamine-induced cystitis (KIC) and ovariectomy-induced overactive bladder (OAB).[51] However, the application to human use is not clear.
Neurotoxicity
Short-term exposure of cultures of GABAergic neurons to ketamine at high concentrations led to a significant loss of differentiated cells in one study, and non-cell death-inducing concentrations of ketamine (10 μg/ml) may still initiate long-term alterations of the dendritic arbor in differentiated neurons.[52][53]
More recent studies of ketamine-induced neurotoxicity have focused on primates in an attempt to use a more accurate model than rodents. One such study administered daily ketamine doses consistent with typical recreational doses (1 mg/kg IV) to adolescent cynomolgus monkeys for varying periods of time.
Decreased locomotor activity and indicators of increased cell death in the prefrontal cortex were detected in monkeys given daily injections for six months, but not those given daily injections for one month.[54]
Dependence and abuse potential
Ketamine has moderate to high abuse potential and produces psychological dependence with chronic use. When dependence has developed, cravings and withdrawal effects may occur if a person suddenly stops their usage.
Tolerance to the main effects of ketamine develops with prolonged and repeated use. This results in users having to administer increasingly large doses to achieve the same effects. Tolerance can decrease after a break (often called a tolerance break), but the duration required to fully reset tolerance varies. This reset period depends on several factors, including an individual’s genetic and physiological makeup, the quantity and frequency of use, and the length of time exposed to the substance.
Ketamine presents cross-tolerance with all dissociatives, meaning that after the consumption of ketamine all dissociatives will have a reduced effect.
Permatolerance
Dissociatives are reported to be unique from other substances because they are capable of producing a long-term or permanent form of tolerance ("permatolerance") that accumulates slowly and independently from normal tolerance.
Many chronic ketamine users report that they need to consume substantially more to achieve dissociation or k-hole compared to their first use, even after taking extended breaks. The cause is not known, although it has been suggested to be a potential indicator of some form of neurotoxicity.
Dissociative permatolerance poses an additional problem considering ketamine's negative effects on the urinary tract. As a result, heavy or chronic use of all dissociatives is strongly discouraged.
Overdose
Fatal ketamine overdoses are rare. Ketamine's toxicity drastically increases when it is mixed with other substances. Overdoses can cause respiratory depression, vomiting, positional asphyxia, heart problems, rhabdomyolysis causing kidney failure and rarely seizures.[55] However, there is evidence that suggests extremely high doses may result in damage to the brain and other organs.[citation needed]
Caution for intravenous use
Rapidly injected ketamine (under 2 minutes) can cause cause transient respiratory depression. The effects of ketamine on reflexes and rapid loss of normal consciousness can increase the chances of positional asphyxia (passing out in a position where you can't breath).[56]
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- Alcohol - Both substances cause ataxia and bring a very high risk of vomiting and unconsciousness. If the user falls unconscious while under the influence there is a severe risk of vomit aspiration if they are not placed in the recovery position.
- GHB / GBL - Both substances cause ataxia and bring a risk of vomiting and unconsciousness. If the user falls unconscious while under the influence there is a severe risk of vomit aspiration if they are not placed in the recovery position.
- Opioids - Both substances bring a risk of vomiting and unconsciousness. If the user falls unconscious while under the influence there is a severe risk of vomit aspiration if they are not placed in the recovery position.
- Tramadol - Tramadol lowers the seizure threshold. Both substances increase the risk of vomiting and unconsciousness.
- Amphetamines - No unexpected interactions, though likely to increase blood pressure (likely not an issue with sensible doses). Moving around on high doses of this combination may be ill-advised due to risk of physical injury.
- Cocaine - No unexpected interactions, though likely to increase blood pressure (likely not an issue with sensible doses). Moving around on high doses of this combination may be ill-advised due to risk of physical injury.
- Benzodiazepines - Both substances potentiate the ataxia and sedation caused by the other and can lead to unexpected loss of consciousness at high doses. While unconscious, vomit aspiration is a risk if the user is not placed in the recovery position.
- MAOIs - MAO-B inhibitors appear to increase the potency of ketamine. MAO-A inhibitors have some negative reports associated with the combination but there isn't much information available
- Trazodone - When used as a sleep aid and taken close to that of a dose of ketamine, there is a risk of respiratory depression when high amounts of either are consumed.
- Grapefruit - Grapefruit juice significantly increases oral absorption of ketamine. This may result in the user having double the concentration of ketamine in their system compared to normal. The ketamine may also have a longer duration of effect.[57] This likely applies to oral, sublingual, and intranasal administration.
Legal status
- Australia: Ketamine is a Schedule 8 drug in Australia, meaning that possession, manufacture or supply without authority is illegal.[58]
- Austria: Ketamine is classed as a NR medication (prescription only, repeated dispense prohibited) and legal for medical and veterinary use,[59] but illegal when sold, possessed or produced without a prescription under the NPSG (Neue-Psychoaktive-Substanzen-Gesetz).[60]
- Belgium: Ketamine is legal for medical and veterinary use and illegal when sold or possessed without a prescription.[citation needed]
- Brazil: Ketamine is legal for veterinary use and illegal when sold or possessed for human use.[citation needed]
- Canada: Ketamine is controlled under Schedule I of the Controlled Drugs and Substances Act[61]. Activities such as the sale, possession or production of ketamine are illegal unless authorized for medical, scientific or industrial purposes. In Canada, ketamine has legitimate uses in medicine.[62]
- China: Ketamine is a Schedule II drug.[citation needed]
- Czech Republic: Ketamine is a Schedule IV [63] (List 7) substance. Sold exclusively with a prescription "without a blue stripe" (§ 1, g), 1. of Nařízení vlády č. 463/2013 Sb.) [64]
- Denmark: Ketamine is legal for medical and veterinary use and illegal when sold or possessed without a prescription.[citation needed]
- France: Ketamine is a Schedule IV drug in France.[citation needed]
- Germany: Ketamine is a prescription medicine, according to Anlage 1 AMVV.[65]
- Hong Kong: Ketamine is a Schedule I drug in Hong Kong.[citation needed]
- Luxembourg: Ketamine is a prohibited substance for recreational use.[66]
- Japan: Ketamine is classified as a narcotic under the Narcotic and Psychotropic Drugs Control Act (麻薬及び向精神薬取締法).[67]
- Malaysia: Ketamine is illegal to sell and possess in Malaysia.[citation needed]
- Mexico: Ketamine is a Category 3 drug in Mexico.[citation needed]
- New Zealand: Ketamine is a Class C drug in New Zealand.[citation needed]
- Norway: Ketamine is a Class A drug in Norway.[citation needed]
- Singapore: Ketamine is a Class A drug in Singapore.[citation needed]
- Slovakia: Ketamine is a Schedule II drug in Slovakia.[citation needed]
- South Korea: Ketamine is illegal to possess and sell in South Korea.[citation needed]
- Spain: Ketamine is a Schedule IV drug in Spain.[68]
- Sweden: Ketamine is a Schedule IV drug in Sweden.[citation needed]
- Switzerland: Ketamine is a controlled substance specifically named under Verzeichnis B, when possessed or handled without a license. Medicinal use is permitted.[69]
- Taiwan: Ketamine is a Schedule III drug in Taiwan.[citation needed]
- Turkey: Ketamine is a 'green prescription' only substance[70] and illegal when sold or possessed without a prescription.[citation needed]
- United Kingdom: Ketamine is a Class B drug in the United Kingdom.[71]
- United States: Ketamine is a Schedule III drug in the United States.[72]
- Poland: Ketamine is illegal to possess, manufacture and sell except for medical purposes. [73]
- Italy: Ketamine is a Schedule I drug in Italy. [74]
See also
External links
- Ketamine (Wikipedia)
- Ketamine (Erowid Vault)
- Ketamine (Isomer Design)
- Ketamine (DrugBank)
- Esketamine (DrugBank)
- Ketamine (Drugs.com)
- Esketamine (Drugs.com)
- Ketamine (Drugs-Forum)
Media
- Interview with a Ketamine Chemist (VICE)
- The Experimental Ketamine Cure for Depression (VICE)
- Ketamine: Dreams and Realities (Jansen 2000, 2004)
Literature
- Durieux, M., & Kohrs, R.T. (1998). Ketamine: teaching an old drug new tricks. Anesthesia and A nalgesia, 87 5, 1186-93. PMID: 9806706
- Mion, G. (2017). History of anaesthesia: The ketamine story–past, present and future. European Journal of Anaesthesiology (EJA), 34(9), 571-575. https://doi.org/10.1097/EJA.0000000000000638
- Krystal, J. H., Karper, L. P., Seibyl, J. P., Freeman, G. K., Delaney, R., Bremner, J. D., . . . Charney, D. S. (1994). Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of General Psychiatry, 51(3), 199-214. http://dx.doi.org/10.1001/archpsyc.1994.03950030035004
- Morris, H., & Wallach, J. (2014). From PCP to MXE: A comprehensive review of the non-medical use of dissociative drugs. Drug Testing and Analysis, 6(7–8), 614–632. https://doi.org/10.1002/dta.1620
References
- ↑ Hall, D., Robinson, A. (September 2014). "INTRANASAL KETAMINE FOR PROCEDURAL SEDATION". Emergency Medicine Journal. 31 (9): 789.2–790. doi:10.1136/emermed-2014-204221.28. ISSN 1472-0205.
- ↑ Clements, J.A.; Nimmo, W.S.; Grant, I.S. (1982). "Bioavailability, Pharmacokinetics, and Analgesic Activity of Ketamine in Humans". Journal of Pharmaceutical Sciences. 71 (5): 539–542. doi:10.1002/jps.2600710516. ISSN 0022-3549.
- ↑ 3.0 3.1 Yanagihara, Y., Ohtani, M., Kariya, S., Uchino, K., Hiraishi, T., Ashizawa, N., Aoyama, T., Yamamura, Y., Yamada, Y., Iga, T. (January 2003). "Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers". Biopharmaceutics & Drug Disposition. 24 (1): 37–43. doi:10.1002/bdd.336. ISSN 0142-2782.
- ↑ Rolan, Paul; Lim, Stephen; Sunderland, Vivian; Liu, Yandi; Molnar, Valeria (2013). "The absolute bioavailability of racemic ketamine from a novel sublingual formulation". British Journal of Clinical Pharmacology. 77 (6): 1011–1016. doi:10.1111/bcp.12264. ISSN 0306-5251.
- ↑ Ketamine: Dreams and Realities, p129, p172
- ↑ Corazza, O., Assi, S., Schifano, F. (June 2013). "From "Special K" to "Special M": The Evolution of the Recreational Use of Ketamine and Methoxetamine". CNS Neuroscience & Therapeutics. 19 (6): 454–460. doi:10.1111/cns.12063. ISSN 1755-5930.
- ↑ Murrough, J. W., Perez, A. M., Pillemer, S., Stern, J., Parides, M. K., Rot, M. aan het, Collins, K. A., Mathew, S. J., Charney, D. S., Iosifescu, D. V. (August 2013). "Rapid and Longer-Term Antidepressant Effects of Repeated Ketamine Infusions in Treatment-Resistant Major Depression". Biological Psychiatry. 74 (4): 250–256. doi:10.1016/j.biopsych.2012.06.022. ISSN 0006-3223.
- ↑ Grof, S. (2010). The ultimate journey: consciousness and the mystery of death (2. ed ed.). MAPS. ISBN 9780966001976.
- ↑ Dalgarno, P. J., Shewan, D. (April 1996). "Illicit Use of Ketamine in Scotland". Journal of Psychoactive Drugs. 28 (2): 191–199. doi:10.1080/02791072.1996.10524391. ISSN 0279-1072.
- ↑ J Arditti (2000) “Ketamine, déviation d’usage en France,” Centre d’Evaluation et d’Information sur la Pharmacodépendance: Marseille, France
- ↑ Klein, M., Kramer, F. (February 2004). "Rave drugs: pharmacological considerations". AANA journal. 72 (1): 61–67. ISSN 0094-6354.
- ↑ Kolp, Eli; Friedman, Harris L.; Krupitsky, Evgeny; Jansen, Karl; Sylvester, Mark; Young, M. Scott; Kolp, Anna (2014-07-01). "Ketamine Psychedelic Psychotherapy: Focus on its Pharmacology, Phenomenology, and Clinical Applications". International Journal of Transpersonal Studies. 33 (2): 93–96. doi:10.24972/ijts.2014.33.2.84. ISSN 1321-0122.
- ↑ Tsai, T.-H., Cha, T.-L., Lin, C.-M., Tsao, C.-W., Tang, S.-H., Chuang, F.-P., Wu, S.-T., Sun, G.-H., Yu, D.-S., Chang, S.-Y. (October 2009). "Ketamine-associated bladder dysfunction". International Journal of Urology. 16 (10): 826–829. doi:10.1111/j.1442-2042.2009.02361.x. ISSN 0919-8172.
- ↑ 14.0 14.1 Morgan, Celia J. A.; Muetzelfeldt, Leslie; Curran, H. Valerie (2010). "Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study". Addiction. 105 (1): 121–133. doi:10.1111/j.1360-0443.2009.02761.x. ISSN 0965-2140.
- ↑ Liang, H.J.; Lau, C.G.; Tang, A.; Chan, F.; Ungvari, G.S.; Tang, W.K. (2013). "Cognitive impairments in poly-drug ketamine users". Addictive Behaviors. 38 (11): 2661–2666. doi:10.1016/j.addbeh.2013.06.017. ISSN 0306-4603.
- ↑ Gao M, Rejaei D, Liu H. Ketamine use in current clinical practice. Acta Pharmacol Sin. 2016 Jul;37(7):865-72. doi: 10.1038/aps.2016.5. Epub 2016 Mar 28. PMID: 27018176; PMCID: PMC4933765.
- ↑ Mion, Georges (2017). "History of anaesthesia". European Journal of Anaesthesiology. 34 (9): 571–575. doi:10.1097/EJA.0000000000000638. ISSN 0265-0215.
- ↑ WHO Model List of Essential Medicines | http://whqlibdoc.who.int/hq/2011/a95053_eng.pdf
- ↑ https://www.deadiversion.usdoj.gov/drug_chem_info/ketamine.pdf
- ↑ 20.0 20.1 20.2 Hashimoto, K. (October 2019). "Rapid-acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective". Psychiatry and Clinical Neurosciences. 73 (10): 613–627. doi:10.1111/pcn.12902. ISSN 1440-1819.
- ↑ Hirota, K., Sikand, K. S., Lambert, D. G. (1 May 1999). "Interaction of ketamine with μ2 opioid receptors in SH-SY5Y human neuroblastoma cells". Journal of Anesthesia. 13 (2): 107–109. doi:10.1007/s005400050035. ISSN 1438-8359.
- ↑ Narita, M., Yoshizawa, K., Aoki, K., Takagi, M., Miyatake, M., Suzuki, T. (September 2001). "A putative sigma 1 receptor antagonist NE-100 attenuates the discriminative stimulus effects of ketamine in rats". Addiction Biology. 6 (4): 373–376. doi:10.1080/13556210020077091. ISSN 1355-6215.
- ↑ Pharmaceutical Society of Australia. "2.1.1 IV general anaesthetics". Australian Medicines Handbook. 2011. Australian Medicines Handbook Pty Ltd. p. 13.
- ↑ Martin, L. L., Bouchal, R. L., Smith, D. J. (February 1982). "Ketamine inhibits serotonin uptake in vivo". Neuropharmacology. 21 (2): 113–118. doi:10.1016/0028-3908(82)90149-6. ISSN 0028-3908.
- ↑ Wang, X., Zhou, Z. J., Zhang, X. F., Zheng, S. (September 2010). "A Comparison of Two Different Doses of Rectal Ketamine Added to 0.5 mg.kg -1 Midazolam and 0.02 mg.kg -1 Atropine in Infants and Young Children". Anaesthesia and Intensive Care. 38 (5): 900–904. doi:10.1177/0310057X1003800515. ISSN 0310-057X.
- ↑ Vargas, MV; Meyer, R; Avanes, AA; Rus, M; Olson, DE (2021). "Psychedelics and Other Psychoplastogens for Treating Mental Illness". Frontiers in psychiatry. 12: 727117. doi:10.3389/fpsyt.2021.727117. PMC 8520991
 Check
Check |pmc=value (help). PMID 34671279. - ↑ Newton, A., Fitton, L. (1 August 2008). "Intravenous ketamine for adult procedural sedation in the emergency department: a prospective cohort study". Emergency Medicine Journal. 25 (8): 498–501. doi:10.1136/emj.2007.053421. ISSN 1472-0205.
- ↑ Grabski, Meryem; McAndrew, Amy; Lawn, Will; Marsh, Beth; Raymen, Laura; Stevens, Tobias; Hardy, Lorna; Warren, Fiona; Bloomfield, Michael; Borissova, Anya; Maschauer, Emily; Broomby, Rupert; Price, Robert; Coathup, Rachel; Gilhooly, David; Palmer, Edward; Gordon-Williams, Richard; Hill, Robert; Harris, Jen; Mollaahmetoglu, O. Merve; Curran, H. Valerie; Brandner, Brigitta; Lingford-Hughes, Anne; Morgan, Celia J.A. (2022). "Adjunctive Ketamine With Relapse Prevention–Based Psychological Therapy in the Treatment of Alcohol Use Disorder". American Journal of Psychiatry. American Psychiatric Association Publishing. 179 (2): 152–162. doi:10.1176/appi.ajp.2021.21030277. ISSN 0002-953X.
- ↑ Ivan Ezquerra-Romano, I.; Lawn, W.; Krupitsky, E.; Morgan, C.J.A. (2018). "Ketamine for the treatment of addiction: Evidence and potential mechanisms". Neuropharmacology. Elsevier BV. 142: 72–82. doi:10.1016/j.neuropharm.2018.01.017. ISSN 0028-3908.
- ↑ 30.0 30.1 Martial, C; Cassol, H; Charland-Verville, V; Pallavicini, C; Sanz, C; Zamberlan, F; Vivot, RM; Erowid, F; Erowid, E; Laureys, S; Greyson, B; Tagliazucchi, E (March 2019). "Neurochemical models of near-death experiences: A large-scale study based on the semantic similarity of written reports". Consciousness and cognition. 69: 52–69. doi:10.1016/j.concog.2019.01.011. PMID 30711788.
- ↑ Jansen K (2001). Ketamine: Dreams and Realities. Multidisciplinary Association for Psychedelic Studies. p. 122. ISBN 978-0-9660019-3-8.
- ↑ "Erowid Ketamine Vault : Ketamine and Quantum Psychiatry, by Karl Jansen". www.erowid.org.
- ↑ Reporter, S. (2012), Ketamine Improves Bipolar Depression Within Minutes
- ↑ Hamilton, J. (2012), Could A Club Drug Offer “Almost Immediate” Relief From Depression?
- ↑ Nishimura, M., Sato, K. (October 1999). "Ketamine stereoselectively inhibits rat dopamine transporter". Neuroscience Letters. 274 (2): 131–134. doi:10.1016/S0304-3940(99)00688-6. ISSN 0304-3940.
- ↑ Zhang JC, Li SX, Hashimoto K (2014). "R (-)-ketamine shows greater potency and longer-lasting antidepressant effects than S (+)-ketamine". Pharmacol. Biochem. Behav. 116: 137–41. doi:10.1016/j.pbb.2013.11.033. PMID 24316345.
- ↑ Tyler, M. W., Yourish, H. B., Ionescu, D. F., Haggarty, S. J. (21 June 2017). "Classics in Chemical Neuroscience: Ketamine". ACS Chemical Neuroscience. 8 (6): 1122–1134. doi:10.1021/acschemneuro.7b00074. ISSN 1948-7193.
- ↑ Zanos, P., Moaddel, R., Morris, P. J., Georgiou, P., Fischell, J., Elmer, G. I., Alkondon, M., Yuan, P., Pribut, H. J., Singh, N. S., Dossou, K. S. S., Fang, Y., Huang, X.-P., Mayo, C. L., Wainer, I. W., Albuquerque, E. X., Thompson, S. M., Thomas, C. J., Zarate Jr, C. A., Gould, T. D. (26 May 2016). "NMDAR inhibition-independent antidepressant actions of ketamine metabolites". Nature. 533 (7604): 481–486. doi:10.1038/nature17998. ISSN 0028-0836.
- ↑ Yang, C., Zhou, W., Li, X., Yang, J., Szewczyk, B., Pałucha-Poniewiera, A., Poleszak, E., Pilc, A., Nowak, G. (May 2012). "A bright future of researching AMPA receptor agonists for depression treatment". Expert Opinion on Investigational Drugs. 21 (5): 583–585. doi:10.1517/13543784.2012.667399. ISSN 1354-3784.
- ↑ Andrade, C. (July 2017). "Ketamine for Depression, 4: In What Dose, at What Rate, by What Route, for How Long, and at What Frequency?". The Journal of Clinical Psychiatry. 78 (7): e852–e857. doi:10.4088/JCP.17f11738. ISSN 1555-2101.
- ↑ https://www.ema.europa.eu/en/medicines/human/EPAR/spravato
- ↑ https://www.obaid.info/pdf/MSDS/S/Spravato-Nasal-Spray.pdf
- ↑ https://www.spravato.com/taking-spravato
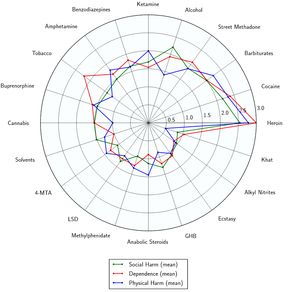
- ↑ Nutt DJ, King LA, Phillips LD (November 2010). "Drug harms in the UK: a multicriteria decision analysis". Lancet. 376 (9752): 1558–1565. CiteSeerX 10.1.1.690.1283
 . doi:10.1016/S0140-6736(10)61462-6. PMID 21036393. Unknown parameter
. doi:10.1016/S0140-6736(10)61462-6. PMID 21036393. Unknown parameter |s2cid=ignored (help) - ↑ Nutt, D., King, L. A., Saulsbury, W., Blakemore, C. (24 March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". The Lancet. 369 (9566): 1047–1053. doi:10.1016/S0140-6736(07)60464-4. ISSN 0140-6736.
- ↑ Kokane, Saurabh S.; Armant, Ross J.; Bolaños-Guzmán, Carlos A.; Perrotti, Linda I. (2020). "Overlap in the neural circuitry and molecular mechanisms underlying ketamine abuse and its use as an antidepressant". Behavioural Brain Research. 384: 112548. doi:10.1016/j.bbr.2020.112548. ISSN 0166-4328.
- ↑ Bozymski, Kevin M.; Crouse, Ericka L.; Titus-Lay, Erika N.; Ott, Carol A.; Nofziger, Jill L.; Kirkwood, Cynthia K. (2019). "Esketamine: A Novel Option for Treatment-Resistant Depression". Annals of Pharmacotherapy. 54 (6): 567–576. doi:10.1177/1060028019892644. ISSN 1060-0280.
- ↑ Gocmen, S., Buyukkocak, U., Caglayan, O. (January 2008). "In Vitro Investigation of the Antibacterial Effect of Ketamine". Upsala Journal of Medical Sciences. 113 (1): 39–46. doi:10.3109/2000-1967-211. ISSN 0300-9734.
- ↑ Middela, S., Pearce, I. (January 2011). "Ketamine-induced vesicopathy: a literature review: Ketamine and bladder". International Journal of Clinical Practice. 65 (1): 27–30. doi:10.1111/j.1742-1241.2010.02502.x. ISSN 1368-5031.
- ↑ Morgan, C. J. A., Curran, H. V., the Independent Scientific Committee on Drugs (ISCD) (January 2012). "Ketamine use: a review: Ketamine use: a review". Addiction. 107 (1): 27–38. doi:10.1111/j.1360-0443.2011.03576.x. ISSN 0965-2140.
- ↑ Jang, M.-Y., Lee, Y.-L., Long, C.-Y., Chen, C.-H., Chuang, S.-M., Lee, H.-Y., Shen, J.-T., Wu, W.-J., Juan, Y.-S. (1 September 2015). "The protective effect of green tea catechins on ketamine-induced cystitis in a rat model". Urological Science. 26 (3): 186–192. doi:10.1016/j.urols.2015.07.010. ISSN 1879-5226.
- ↑ Vutskits, L., Gascon, E., Potter, G., Tassonyi, E., Kiss, J. Z. (20 May 2007). "Low concentrations of ketamine initiate dendritic atrophy of differentiated GABAergic neurons in culture". Toxicology. 234 (3): 216–226. doi:10.1016/j.tox.2007.03.004. ISSN 0300-483X.
- ↑ Hargreaves, R. J., Hill, R. G., Iversen, L. L. (1994). "Neuroprotective NMDA antagonists: the controversy over their potential for adverse effects on cortical neuronal morphology". Acta Neurochirurgica. Supplementum. 60: 15–19. doi:10.1007/978-3-7091-9334-1_4.
- ↑ Sun, L., Li, Q., Li, Q., Zhang, Y., Liu, D., Jiang, H., Pan, F., Yew, D. T. (March 2014). "Chronic ketamine exposure induces permanent impairment of brain functions in adolescent cynomolgus monkeys: Ketamine and brain deficits". Addiction Biology. 19 (2): 185–194. doi:10.1111/adb.12004. ISSN 1355-6215.
- ↑ https://www.ncbi.nlm.nih.gov/books/NBK541087/
- ↑ https://www.ncbi.nlm.nih.gov/books/NBK470357/
- ↑ Peltoniemi, M. A., Saari, T. I., Hagelberg, N. M., Laine, K., Neuvonen, P. J., Olkkola, K. T. (June 2012). "S-ketamine concentrations are greatly increased by grapefruit juice". European Journal of Clinical Pharmacology. 68 (6): 979–986. doi:10.1007/s00228-012-1214-9. ISSN 1432-1041.
- ↑ Health, Poisons Standard February 2019
- ↑ RIS - Rezeptpflichtverordnung - Bundesrecht konsolidiert, Fassung vom 22.07.2022
- ↑ RIS - Neue-Psychoaktive-Substanzen-Gesetz - Bundesrecht konsolidiert, Fassung vom 22.07.2022
- ↑ Branch, L. S. (2022), Consolidated federal laws of Canada, Controlled Drugs and Substances Act
- ↑ Canada, H. (2012), Ketamine
- ↑ https://www.unodc.org/pdf/convention_1971_en.pdf
- ↑ https://www.zakonyprolidi.cz/cs/2013-463
- ↑ AMVV - Verordnung über die Verschreibungspflicht von Arzneimitteln
- ↑ Legilux
- ↑ "麻薬及び向精神薬取締法" [Narcotic and Psychotropic Drugs Control Act] (in Japanese). 厚生労働省 [Ministry of Health, Labour and Welfare]. Retrieved June 7, 2023.
- ↑ BOE.es - BOE-A-1977-27160 Real Decreto 2829/1977, de 6 de octubre por el que se regulan las sustancias y preparados medicinales psicotrópicos, así como la fiscalización e inspección de su fabricación, distribución, prescripción y dispensación.
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
- ↑ YEŞİL REÇETEYE TABİ İLAÇLAR | https://www.titck.gov.tr/storage/Archive/2019/contentFile/01.04.2019%20SKRS%20Ye%C5%9Fil%20Re%C3%A7eteli%20%C4%B0la%C3%A7lar%20Aktif%20SON%20-%20G%C3%9CNCEL_58b1ff4a-2e1c-4867-bad7-eec855d6162a.pdf
- ↑ Drugs penalties, GOV.UK, 3 September 2016. Retrieved on 25 November 2017.
- ↑ [1], DEA.GOV,
- ↑ Wykaz środków odurzających i substancji psychotropowych, 2022
- ↑ https://www.normattiva.it/uri-res/N2Ls?urn:nir:stato:legge:2014;79