3-FEA
| Summary sheet: 3-FEA |
| 3-FEA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common names | 3-FEA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substitutive name | 3-Fluoroethamphetamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Systematic name | N-Ethyl-1-(3-fluorophenyl)propan-2-amine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Psychoactive class | Entactogen / Stimulant | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical class | Amphetamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alcohol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GHB | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GBL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Opioids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cocaine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cannabis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Caffeine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ketamine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Methoxetamine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Psychedelics | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DXM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PCP | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 25x-NBOMe | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2C-T-x | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5-MeO-xxT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DOx | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tramadol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| aMT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MAOIs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3-Fluoroethamphetamine (also known as 3-FEA) is a novel ring-substituted amphetamine compound that produces a mixture of entactogenic and stimulant effects when administered. 3-FEA is structurally related to a series of designer fluorinated substituted amphetamines that originally included compounds such as 2-FA, 2-FMA, 3-FA, 4-FMA, 4-FA.[1]
Like its parent compound 3-FA, the pharmacological, toxicological, and subjective effects of 3-FEA in humans have yet to be mapped out in detail. Anecdotal reports have characterised 3-FEA as a moderately potent serotonin-dominant triple monoamine releaser that produces a mixture of entactogenic and mild stimulating effects.[citation needed]
3-FEA has an extremely short history of human recreational use and has not been documented being sold on the streets. It has recently been made available for sale on the grey market as a research chemical by online vendors.[citation needed] Due to its potent psychostimulant effects, likely habit-forming properties, and unknown toxicity profile, it is strongly recommended that one use proper harm reduction practices if using this substance.
Chemistry
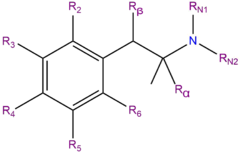
3-FEA, or 3-fluoroethamphetamine, is a synthetic molecule of the amphetamine chemical class. Molecules of the amphetamine class contain a phenethylamine core comprised of a phenyl ring bound to an amino (NH2) group through an ethyl chain substituted with a methyl group at Rα (i.e. amphetamines are alpha-methylated phenethylamines).
3-FEA is the 3-position fluorinated analog of ethylamphetamine (also known as ethamphetamine). It is also an analog of fenfluramine with the 3-trifluoromethyl group replaced with a 3-fluoro substituent.[citation needed]
Pharmacology
Although 3-FEA has not been formally studied on the same level as traditional amphetamines, it is currently assumed that like other substituted amphetamines with substitutions at similar positions, it most likely acts primarily as a triple reuptake inhibitor and/or releaser of the monoamine neurotransmitters serotonin, dopamine, and norepinephrine.[2][3]
It has been demonstrated that compared to the unsubstituted ethylamphetamine, 3-fluoroethamphetamine is a weaker releaser of dopamine, but a stronger releaser of both serotonin and norepinephrine, producing the strongest reinforcing effects in animal studies out of a range of 3-substituted amphetamine derivatives tested, despite not being the most potent dopamine releaser.[3][2]
This indicates that 3-FEA effectively increases the levels of all the three major monoamine neurotransmitters dopamine, norepinephrine, and serotonin in the brain by acting as a releasing agent of said neurotransmitters and/or by binding to and partially blocking the transporter proteins that normally clear those molecules from the synaptic cleft after they have fulfilled their function of conducting a neural impulse. This transporter blockade allows these molecules to accumulate within core synaptic regions of the brain to extra-endogenous levels, resulting in a combination of relaxing, stimulating, disinhibiting and euphoric effects associated with entactogenic substituted amphetamines such as MDMA or 4-FA.[citation needed]
Subjective effects
Unlike its close analog 3-FA, which has been reported as being relatively functional and non-recreational, 3-FEA appears to produce effects more similar to another analog 4-FA, which produces marked entactogenic effects. 3-FEA has also been reported to produce less stimulation compared to 4-FA to the degree that some users report it as being primarily sedating. This effect profile likely makes 3-FEA a poor candidate for functional use and better suited for recreational use in a manner similar to MDMA.
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Sedation & Stimulation - 3-FEA has been reported to produce a paradoxical combination of sedating and stimulatory effects, with the sedating aspects believed to be attributable to the significant amount of serotonin it releases. The stimulatory effects are typically more prominent at dosages in and above the strong dosage range and tend to become prominent after the peak effects of a dose have subsided.
- Perception of bodily heaviness - Depending on whether one is feeling more stimulated than sedated, one will often feel as if they are lighter (as in this case) or heavier in the other.
- Spontaneous bodily sensations - Early reports have described the "body high" of 3-FEA as a moderate to strong euphoric tingling sensation that can encompass the entire body that is capable of becoming extremely pleasurable at higher dosages. This sensation maintains a consistent presence that steadily rises with the onset and hits its limit once the peak has been reached.
- Physical euphoria
- Tactile enhancement - This component primarily tends to occur with higher doses.
- Abnormal heartbeat[citation needed]
- Increased heart rate[citation needed]
- Increased blood pressure[citation needed]
- Increased perspiration
- Temperature regulation suppression[citation needed]
- Headaches
- Vasoconstriction[citation needed]
- Dehydration
- Dry mouth[citation needed]
- Difficulty urinating - 3-FEA can result in difficulty with urination, an effect that is usually temporary and that tends to become more likely and more significant with higher dosages.
- Appetite suppression - This effect can reportedly continue long after the duration of the main effects of the substance have subsided.[citation needed]
- Pupil dilation - This effect is often very significant at even lower common dosages and the significance at common dosages has been described as being in line with that of a standard dosage of MDMA. This effect can also reportedly continue long after the duration of the main effects of the substance have subsided.[citation needed] The significance of this effect is thought to be attributable to the significant amount of serotonin that 3-FEA releases.[citation needed]
- Teeth grinding - Teeth grinding often occurs with higher or multiple doses and can be comparable in extent as MDMA.
- Diarrhea - 3-FEA has been reported to produce a laxative effect in some users.[citation needed]
Cognitive effects 
-
- Anxiety suppression or Anxiety - 3-FEA typically produces anxiety suppressing effects characteristic of serotonin-releasing entactogens; however, anxiety may also occur nearer the end of the substance's effect duration when higher dosages have been used.
- Disinhibition - 3-FEA produces a similar degree of disinhibition as MDMA.
- Empathy, affection, and sociability enhancement - This effect is reportedly very significant, with dosages in the common range and above often reportedly having this effect occur at a significance in line with that of a common dosage of MDMA.[citation needed]
- Ego inflation
- Thought acceleration or Thought deceleration - 3-FEA can often case thought acceleration during the come-up and first half of the peak of the substance's effect duration, but then may also cause minor to significant thought deceleration from the latter half of the peak onward, typically more often at higher dosages.
- Cognitive euphoria
- Increased music appreciation - This effect is often reported as being a prominent effect at all dosages.[citation needed]
- Increased libido
- Immersion enhancement
- Motivation enhancement
- Compulsive redosing - This effect is often most prominent during or immediately following the peak of the substance's effects.
- Time distortion - This can be described as the experience of time speeding up and passing much quicker than it usually would when sober.
- Wakefulness - This effect typically only occurs following the use of higher or multiple dosages.
After effects 
- The effects which occur during the offset of a stimulant experience generally feel negative and uncomfortable in comparison to the effects which occurred during its peak. This is often referred to as a "comedown" and occurs because of neurotransmitter depletion. Its effects commonly include:
Experience reports
Anecdotal reports which describe the effects of this compound within our experience index include:
Additional experience reports can be found here:
Toxicity and harm potential
The toxicity and long-term health effects of recreational 3-FEA use do not seem to have been studied in any scientific context and the exact toxic dosage is unknown. This is because 3-FEA has an extremely brief history of human usage, first becoming available in mid-2016.
Early anecdotal reports suggest that there do not seem to be any negative health effects attributed to simply trying this substance at low to moderate doses by itself and using it very cautiously sparingly (although nothing can be completely guaranteed).
Due to its serotonin-releasing entactogenic properties, it is possible 3-FEA may display significant affinity and activity at the 5-HT2B receptor, which like 5-HT2B agonists such as MDMA and fenfluramine would make it cardiotoxic with long-term or heavy use.[4]
It is strongly recommended that one use proper harm reduction practices when using this substance.
Tolerance and addiction potential
Although it still remains to be seen, the chronic use of 3-FEA will likely come to be considered to be moderately addictive with a high potential for abuse and capable of causing psychological dependence among a certain population of users. When dependence or addiction has developed, cravings and withdrawal effects may occur if a person suddenly stops their usage.
Tolerance to many of the effects of 3-FEA develops with prolonged and repeated use. This results in users having to administer increasingly large doses to achieve the same effects. Afterward, it takes about 2 - 3 days for the tolerance to be reduced to half and 3-7 days to be back at baseline (in the absence of further consumption). 3-FEA likely presents cross-tolerance with all dopaminergic and serotonergic stimulants and entactogens, meaning that after the consumption of 3-FEA all stimulants will have a reduced effect (including atypical stimulants one might not expect, such MDMA or amphetamine due to its reliance on robust dopamine and norepinephrine stores to exert its full spectrum of effect).
Psychosis
Abuse of compounds within the amphetamine chemical class at high dosages for prolonged periods of time can potentially result in a stimulant psychosis that may present with a variety of symptoms (e.g., paranoia, hallucinations, or delusions).[5] A review on treatment for amphetamine, dextroamphetamine, and methamphetamine abuse-induced psychosis states that about 5–15% of users fail to recover completely.[5][6] The same review asserts that, based upon at least one trial, antipsychotic medications effectively resolve the symptoms of acute amphetamine psychosis.[5] Psychosis very rarely arises from therapeutic use.[7]
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- Alcohol - Drinking alcohol on stimulants is considered risky because it reduces the sedative effects of the alcohol that the body uses to gauge drunkenness. This often leads to excessive drinking with greatly reduced inhibitions, increasing the risk of liver damage and increased dehydration. The effects of stimulants will also allow one to drink past a point where they might normally pass out, increasing the risk. If you do decide to do this then you should set a limit of how much you will drink each hour and stick to it, bearing in mind that you will feel the alcohol and the stimulant less.
- GHB/GBL - Stimulants increase respiration rate allowing a higher dose of sedatives. If the stimulant wears off first then the depressant effects of the GHB/GBL may overcome the user and cause respiratory arrest.
- Opioids - Stimulants increase respiration rate allowing a higher dose of opiates. If the stimulant wears off first then the opiate may overcome the patient and cause respiratory arrest.
- Cocaine - The rewarding effects of cocaine are mediated by DAT inhibition, and an increase of exocytosis of dopamine through the cell membrane. Amphetamine reverses the direction of DAT and the direction vesicular transports within the cell by a pH mediated mechanism of displacement, thus excludes the regular mechanism of dopamine release through means of exocytosis because the effects Na+/K+ ATPase are inhibited. You will find cardiac effects with the combination of cocaine and amphetamine due to a SERT mediated mechanism from the subsequent activation of 5-HT2B, which is an effect of serotonin-related valvulopathy. Amphetamines generally cause hypertension in models of abuse, and this combination can increase the chances of syncope due to turbulent blood flow during valve operation. The rewarding mechanisms of cocaine are reversed by administration of amphetamine.[8][9]
- Cannabis - Stimulants increase anxiety levels and the risk of thought loops and paranoia which can lead to negative experiences.
- Caffeine - This combination of stimulants is generally considered unnecessary and may increase strain on the heart, as well as potentially causing anxiety and physical discomfort.
- Tramadol - Tramadol and stimulants both increase the risk of seizures.
- DXM - Both substances raise heart rate, in extreme cases, panic attacks caused by these substances have led to more serious heart issues.
- Ketamine - Combining amphetamine and ketamine may result in psychoses that resemble schizophrenia, but not worse than the psychoses produced by either substance alone, but this is debatable. This is due to amphetamines ability to attenuated the disruption of working memory caused by ketamine. Amphetamine alone may result in grandiosity, paranoia, or somatic delusions with little to no effect on negative symptoms. Ketamine, however, will result in thought disorders, disruption of executive functioning, and delusions due to a modification of conception. These mechanisms are due to an increase of dopaminergic activity in the mesolimbic pathway caused by amphetamine due to its pharmacology effecting dopamine, and due to a disruption of dopaminergic functioning in the mesocortical pathways via NMDA antagonism effects of ketamine. Combining the two, you may expect mainly thought disorder along with positive symptoms.[10]
- PCP - Increases risk of tachycardia, hypertension, and manic states.
- Methoxetamine - Increases risk of tachycardia, hypertension, and manic states.
- Psychedelics (e.g. LSD, mescaline, psilocybin) - Increases risk of anxiety, paranoia, and thought loops.
- 25x-NBOMe - Amphetamines and NBOMes both provide considerable stimulation that when combined they can result in tachycardia, hypertension, vasoconstriction and, in extreme cases, heart failure. The anxiogenic and focusing effects of stimulants are also not good in combination with psychedelics as they can lead to unpleasant thought loops. NBOMes are known to cause seizures and stimulants can increase this risk.
- 2C-T-x - Suspected of mild MAOI properties. May increase the risk of hypertensive crisis.
- 5-MeO-xxT - Suspected of mild MAOI properties. May increase the risk of hypertensive crisis.
- DOx
- aMT - aMT has MAOI properties which may interact unfavorably with amphetamines.
- MAOIs - MAO-B inhibitors can increase the potency and duration of phenethylamines unpredictably. MAO-A inhibitors with amphetamine can lead to hypertensive crises.
Legal status
3-FEA is currently a grey area compound within many parts of the world, meaning its regulation lies in a legal grey area and that it is not known to be specifically illegal ("scheduled") within any country. However, individuals may still be charged for its possession under certain circumstances such as under analog laws and with intent to sell or consume.
- Austria: 3-FEA is illegal to produce and sell under the NPSG (Neue-Psychoaktive-Substanzen-Gesetz Österreich).[citation needed]
- Canada: 3-FEA would be considered Schedule I as it is an analog of Amphetamine.[11]
- France: As of december 2024, 3-FEA is not explicitly scheduled. It is thus legal to possess, although in a grey area.[12]
- Germany: 3-FEA is controlled under the NpSG (New Psychoactive Substances Act)[13] as of November 26, 2016.[14] Production and import with the aim to place it on the market, administration to another person and trading is punishable. Possession is illegal but not penalized.[15]
- New Zealand: 3-FEA is an amphetamine analog, so is a Schedule 3 controlled substance in New Zealand.[16]
- Switzerland: 3-FEA can be considered a controlled substance as a defined derivative of a-methylphenethylamine under Verzeichnis E point 130. It is legal when used for scientific or industrial use.[17]
- United Kingdom: 3-FEA is considered a Class A drug as a result of the amphetamine analog clause of the Misuse of Drugs Act 1971.[18]
- United States: 3-FEA may be considered to be an analogue of amphetamine under the Federal Analogue Act and thus a Schedule II drug. The Federal Analogue Act, 21 U.S.C. § 813, is a section of the United States Controlled Substances Act, allowing any chemical "substantially similar" to an illegal drug (in Schedule I or II) to be treated as if it were also in Schedule I or II, but only if it is intended for human consumption.[citation needed]
See also
External links
References
- ↑ Rösner, P., Quednow, B., Girreser, U., Junge, T. (March 2005). "Isomeric Fluoro-methoxy-phenylalkylamines: a new series of controlled-substance analogues (designer drugs)". Forensic Science International. 148 (2–3): 143–156. doi:10.1016/j.forsciint.2004.05.003. ISSN 0379-0738.
- ↑ 2.0 2.1 Tessel, R. E., Rutledge, C. O. (May 1976). "Specificity of release of biogenic amines from isolated rat brain tissue as a function of the meta substituent of N-ethylamphetamine derivatives". The Journal of Pharmacology and Experimental Therapeutics. 197 (2): 253–262. ISSN 0022-3565.
- ↑ 3.0 3.1 Tessel, R. E., Woods, J. H. (May 1978). "Substituted N-ethylamphetamine self injection responding in the rhesus monkey: structure-activity relationships". The Journal of Pharmacology and Experimental Therapeutics. 205 (2): 274–281. ISSN 0022-3565.
- ↑ Rothman, R. B., Baumann, M. H., Savage, J. E., Rauser, L., McBride, A., Hufeisen, S. J., Roth, B. L. (5 December 2000). "Evidence for Possible Involvement of 5-HT 2B Receptors in the Cardiac Valvulopathy Associated With Fenfluramine and Other Serotonergic Medications". Circulation. 102 (23): 2836–2841. doi:10.1161/01.CIR.102.23.2836. ISSN 0009-7322.
- ↑ 5.0 5.1 5.2 Shoptaw, S. J., Kao, U., Ling, W. (21 January 2009). Cochrane Drugs and Alcohol Group, ed. "Treatment for amphetamine psychosis". Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD003026.pub3. ISSN 1465-1858.
- ↑ Hofmann, F. G. (1983). A handbook on drug and alcohol abuse: the biomedical aspects (2nd ed ed.). Oxford University Press. ISBN 9780195030563.
- ↑ http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf
- ↑ Greenwald, M. K., Lundahl, L. H., Steinmiller, C. L. (December 2010). "Sustained Release d-Amphetamine Reduces Cocaine but not 'Speedball'-Seeking in Buprenorphine-Maintained Volunteers: A Test of Dual-Agonist Pharmacotherapy for Cocaine/Heroin Polydrug Abusers". Neuropsychopharmacology. 35 (13): 2624–2637. doi:10.1038/npp.2010.175. ISSN 0893-133X.
- ↑ Siciliano, C. A., Saha, K., Calipari, E. S., Fordahl, S. C., Chen, R., Khoshbouei, H., Jones, S. R. (10 January 2018). "Amphetamine Reverses Escalated Cocaine Intake via Restoration of Dopamine Transporter Conformation". The Journal of Neuroscience. 38 (2): 484–497. doi:10.1523/JNEUROSCI.2604-17.2017. ISSN 0270-6474.
- ↑ Krystal, J. H., Perry, E. B., Gueorguieva, R., Belger, A., Madonick, S. H., Abi-Dargham, A., Cooper, T. B., MacDougall, L., Abi-Saab, W., D’Souza, D. C. (1 September 2005). "Comparative and Interactive Human Psychopharmacologic Effects of Ketamine and Amphetamine: Implications for Glutamatergic and Dopaminergic Model Psychoses and Cognitive Function". Archives of General Psychiatry. 62 (9): 985. doi:10.1001/archpsyc.62.9.985. ISSN 0003-990X.
- ↑ Branch, L. S. (2022), Consolidated federal laws of Canada, Controlled Drugs and Substances Act
- ↑ Arrêté du 22 février 1990 fixant la liste des substances classées comme stupéfiants
- ↑ "Anlage NpSG" (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 19, 2019.
- ↑ "Gesetz zur Bekämpfung der Verbreitung neuer psychoaktiver Stoffe" (PDF) (in German). Bundesanzeiger Verlag. Retrieved December 19, 2019.
- ↑ "§ 4 NpSG" (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 19, 2019.
- ↑ Misuse of Drugs Act 1975 No 116 (as at 01 July 2022), Public Act – New Zealand Legislation
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
- ↑ Misuse of Drugs Act 1971