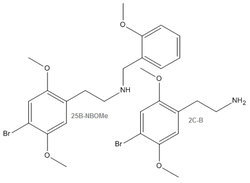
25B-NBOMe

25B-NBOMe can be fatal at heavy doses.[1]
It is strongly discouraged to take large amounts of this substance or to insufflate (snort) it. Please see this section for more details.
| Summary sheet: 25B-NBOMe |
| 25B-NBOMe | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common names | 25B-NBOMe | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substitutive name | 2C-B-NBOMe, BOM 2-CB, Cimbi-36, New Nexus, Nova | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Systematic name | 2-(4-Bromo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl) methyl]ethanamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Psychoactive class | Psychedelic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical class | Phenethylamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2C-T-X | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5-MeO-xxt | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Caffeine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cannabis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DOx | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MAOIs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MDMA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MXE | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Amphetamines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| aMT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cocaine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DXM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tramadol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lithium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
25B-NBOMe (also known as Cimbi-36, NBOMe-2C-B and 2C-B-NBOMe) is novel psychedelic substance of the phenethylamine class. It is a member of the 25x-NBOMe series, a recently discovered group of potent psychedelic compounds derived from the 2C-x family. The name 25B-NBOMe, which is short-hand for 2C-B-NBOMe, indicates it is a derivative of the phenethylamine psychedelic 2C-B.
25B-NBOMe was discovered in 2004 by Ralf Heim at the Free University of Berlin.[2] It acts as a potent partial agonist for the 5-HT2A receptor.[3] It has been used in clinical trials with an evaluation dose for safety consideration to humans of only 1 microgram. Such a dose was determined to be only 1/300th the dose expected to be hallucinogenic to humans and that recreational use would greatly exceed doses determined to be safe to humans.[3] This substance had no history of human use before being sold online as a designer drug in 2010.[citation needed]
Subjective effects include stimulation, open and closed-eye visuals, time distortion, euphoria, and ego loss. Anecdotal reports suggest 25B-NBOMe to be an active hallucinogen at a dose of as little as 250–500 µg, making it a similar potency to other phenethylamine derived hallucinogens such as Bromo-DragonFLY.[4] It is worth noting that compounds of the NBOMe class are not orally active and should therefore be taken sublingually by placing them into one's mouth and allowing it to absorb over a period of 15-30 minutes.
Extremely little is known about the pharmacological properties, metabolism, and toxicity of 25B-NBOMe in humans. Numerous members of the 25x-NBOMe series have been associated with hospitalizations and deaths. Along with its highly sensitive dose-response and unpredictable effects, many reports also suggest that this substance may be overly difficult to use safely. Therefore it is highly advised to use harm reduction practices if using this substance.
Chemistry
25B-NBOMe or 2C-B-NBOMe is a serotonergic N-benzyl derivative of the substituted phenethylamine psychedelic known as 2C-B. 25B-NBOMe is a substituted phenethylamine with methoxy groups CH3O- attached to carbons R2 and R5 as well as a bromine atom attached to carbon R4. It differs from 2C-B structurally through a substitution on the amine (NH2) with a 2-methoxybenzyl (BOMe) group. 25B-NBOMe shares this 2-methoxybenzyl substitution with other chemicals of the NBOMe family. This NBOMe addition contains a methoxy ether CH3O- bound to a benzene ring at R2.
The compound has a density of 1.3 (±0.1) g/cm^3.
Pharmacology
25B-NBOMe has efficacy at the 5-HT2A receptor where it acts as a potent partial agonist.[2][5][6][3] However, the role of these interactions and how they result in the psychedelic experience continues to remain elusive.
Subjective effects
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Stimulation - In terms of its effects on the physical energy levels of the user, 25B-NBOMe is usually considered to be energetic and stimulating, but it can be considered less stimulating when compared to 25I-NBOMe. For most people, this substance induces a unique type of physical stimulation which can be described as feeling extremely energetic but in a way which does not force the person to move unless they genuinely choose to do so. For others, however, the stimulation can be quite uncontrollable, occasionally resulting in bodily shakes and a grinding of the teeth comparable to that of MDMA and traditional stimulants such as amphetamine, but this is manifested much less consistently when compared to that of 25I-NBOMe
- Mouth numbing - Assuming the substance has been taken sublingually, the very first physical effect which a person will notice immediately after sublingual absorption is a strong, unpleasant metallic chemical taste. This is accompanied by a very obvious feeling of general numbness of the tongue and mouth which can stay for up to an hour after the blotter paper has been consumed. This is a key difference when it comes to determining whether one's blotter paper contains LSD or one of the NBOMe series.
- Spontaneous physical sensations - The "body high" itself can be described as a generally mild, all-encompassing, soft but euphoric tingling sensation. This tingling sensation is also accompanied by spontaneous rushes of euphoria that become longer and more drawn out proportional to the dosage consumed.
- Perception of bodily lightness - In terms of the body’s perceived weight, this substance consistently leaves people feeling extremely light, often to the point of total weightlessness.
- Nausea - As the user begins to come up, nausea is not uncommon and can sometimes result in initial vomiting, but passes once this has either happened or the trip begins to fully set in. In comparison to other psychedelics such as psilocin, LSD, 2C-E and 2C-I, this could actually be very considered very mild in its intensity.
- Vasoconstriction
- Increased heart rate
- Pupil dilation
Visual effects 
-
Enhancements
Distortions
- Drifting (melting, flowing, breathing and morphing) - In comparison to other psychedelics, this effect can be described as highly detailed, slow and smooth in motion, static in appearance and unrealistic/cartoon-like in style.
- Tracers
- After images
- Symmetrical texture repetition
- Colour shifting
- Scenery slicing
Geometry
The visual geometry that is present throughout this trip is often described as similar in appearance to that of LSD. They can be comprehensively described as algorithmic in geometric style, intricate in complexity, fine and zoomed out in detail, fast and smooth in motion, structured in shape, colourful in scheme, glossy in colour, sharp around the edges and mostly rounded across their corners. In comparison to other more commonly used psychedelics they can be described as significantly more intricate than the visual geometry found within 2C-I and most of the 2C-x family in general as well as completely on par with LSD, psilocin and DMT at appropriately high dosages.
In terms of their behaviour, 25B-NBOMe’s geometry leads onto Level 8A visual geometry with Level 8B remaining so far unconfirmed within this substance. They also seem to consistently build up in visual intensity when the user stares at a central point. This eventually envelops the visual field and creates the sensation that the user has broken through into a continuously shifting geometric landscape or structure with a vast sense of immersive physical size attributed to it.
Hallucinatory states
- Transformations
- Internal hallucination - (autonomous entities; settings, sceneries, and landscapes; perspective hallucinations and scenarios and plots) These are more common within dark environments and can be described as internal in their manifestation, lucid in believability, interactive in style and almost exclusively of a personal, religious, spiritual, science-fiction, fantasy, surreal, nonsensical or transcendental nature in their overall theme.
Cognitive effects 
-
- Empathy, love and sociability enhancement - The entactogenic effects range from mild to powerful, but are inconsistently manifested. Entactogenic effects for people who try this substance usually become prominent in the presence of others. These feelings of increased sociability, love and empathy do not seem to be quite as strong or profound as those found within other entactogens (such as MDMA, 2C-B and AMT). They are, however, the most prominent entactogenic effects found within the NBOMe series.
- Analysis enhancement - This component is consistently manifested only in the context of a non-social setting in which the user is alone and is introspection dominant.
- Thought acceleration
- Wakefulness
- Time distortion
- Novelty enhancement
- Conceptual thinking
- Thought connectivity
- Emotion enhancement
- Increased music appreciation
- Personal bias suppression
- Memory suppression
- Immersion enhancement
- Increased libido
Auditory effects 
Experience reports
There are currently no anecdotal reports which describe the effects of this compound within our experience index. Additional experience reports can be found here:
Toxicity and harm potential
25B-NBOMe is a relatively new substance, and little is known about its pharmacological risks or its interaction with other substances. The lethal dosage has not yet been determined. One case has been reported on where 25B-NBOMe was identified as the cause of death for a 17-year-old boy.[7]
It is advised that due to 25B-NBOMe's extreme potency it should not be insufflated as this method of administration is potentially fatal at heavy dosages.[1]
25B-NBOMe has been used in clinical trials with an evaluation dose for safety consideration to humans of only 1 microgram; Such a dose is 300× lower than the dose expected to be hallucinogenic to humans and it is expected that recreational use would greatly exceed doses determined to be safe to humans.[8]
It is strongly recommended that one use harm reduction practices when using this substance.
Tolerance and addiction potential
25B-NBOMe is not habit-forming, and the desire to use it can actually decrease with use. It is most often self-regulating.
Tolerance to the effects of 25B-NBOMe is built almost immediately after ingestion. After that, it takes about 1 week for the tolerance to be reduced to half and 2 weeks to be back at baseline (in the absence of further consumption). 25B-NBOMe presents cross-tolerance with all psychedelics, meaning that after the consumption of 25B-NBOMe all psychedelics will have a reduced effect.
Overdose
Due to the very high potency and seemingly unpredictable effects the margin between a normal and an overdose of NBOMe compounds is extremely small when compared to many other substances. The exact toxic dose is unclear since it seems to depend a lot on personal physiology, rather than predominantly dose. However, various anecdotal reports suggest that dangerous side effects begin to appear when exceeding 1000 μg and it possibly becoming lethal for the more sensitive people at roughly 2000 μg. Reports of other people surviving much higher doses, sometimes even without any major side effects have been documented as well.
There is also the uncertainty of dosage on blotter paper since it is rather difficult to lay such an exact dosage. Insufflating, vaporizing or drinking tinctures of this substance is highly discouraged because of this and has been tied to many documented deaths[9][10][1]. One study found that 25I‐NBOMe and 25C‐NBOMe blotter papers contained 'hotspots' with higher quantities of the drug, implying an inherent risk of overdosing.[11]
The overdose effects of NBOMes are typically a dangerously high heart rate, blood pressure, hyperthermia and significant vasoconstriction[12][13] also accompanied by confusion, delusions, panic attacks, aggressive behavior, numbness or pain, amnesia and often seizures. The risks in an overdose include anything from organ failure to cardiac arrest and death[citation needed]. There are also multiple reports of people lethally injuring themselves or falling to death[14][15]. Benzodiazepines or antipsychotics can help with the psychological effects during an overdose although medical attention should always be called in even a possible overdose of 25I-NBOMe.
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit. Due to the highly unpredictable nature of the NBOMe series, it is generally advised to avoid mixing them with other psychoactive substances.
- 2C-T-X - The 2C-T-X phenethylamines can be unpredictable in their interactions and the NBOMes are known to be unpredictable even alone. As a result, this combination should be avoided.
- 5-MeO-xxt - The 5-MeO tryptamines can be unpredictable in their interactions and the NBOMes are known to be unpredictable even alone. As a result, this combination should be avoided.
- Amphetamines - Amphetamines and NBOMes both provide considerable stimulation. When combined they can result in tachycardia, hypertension, vasoconstriction and, in extreme cases, heart failure. The anxiogenic and focusing effects of stimulants are also not good in combination with psychedelics as they can lead to unpleasant thought loops. NBOMes are known to cause seizures and stimulants can increase this risk.
- aMT
- Caffeine - Caffeine can bring out the natural stimulation from psychedelic drugs to make it uncomfortable. High doses can cause anxiety which is hard to handle while tripping.
- Cannabis - Cannabis has an unexpectedly strong and unpredictable synergy with the effects of psychedelics. Caution is advised with this combination as it can significantly increase the risk of adverse psychological reactions like anxiety, paranoia, panic attacks, and psychosis. Users are advised to start off with only a fraction of their normal cannabis dose and take long breaks between hits to avoid over intake.
- Cocaine - Cocaine and NBOMes both provide considerable stimulation. When combined they can result in severe vasoconstriction, tachycardia, hypertension, and in extreme cases heart failure.
- DOx
- DXM
- Lithium - Lithium is commonly prescribed in the treatment of [https://en.wikipedia.org/wiki/Bipolar_disorder bipolar disorder. There is a large body of anecdotal evidence that suggests taking it with psychedelics significantly increases the risk of psychosis and seizures. As a result, this combination is strictly discouraged.
- MAOIs - MAO-B inhibitors can increase the potency and duration of phenethylamines unpredictably.
- MDMA
- MXE - As an NMDA antagonist, MXE potentiates NBOMes which can be unpleasantly intense.
- Tramadol - Tramadol is well known to lower seizure threshold and NBOMes have also shown a tendency to cause severe seizures
Legal status
- Austria: Since June 26, 2019, 25B-NBOMe is illegal to possess, produce and sell under the SMG. (Suchtmittelgesetz Österreich)[16]
- Brazil: Possession, production and sale is illegal as it is listed on Portaria SVS/MS nº 344.[17]
- Canada: 25B-NBOMe would be considered Schedule III as it is a derivative of 2,5-dimethoxyphenethylamine.[18]
- China: As of October 2015, 25B-NBOMe is a controlled substance in China.[19]
- Germany: 25B-NBOMe is controlled under Anlage I BtMG (Narcotics Act, Schedule I) as of December 13, 2014.[20][21] It is illegal to manufacture, possess, import, export, buy, sell, procure or dispense it without a license.[22]
- Italy: 25B-NBOMe is a Schedule 1 controlled substance in Italy.[23]
- Japan: 25B-NBOMe is a narcotic drug in Japan effective November 1st, 2015.[24]
- Latvia: 25B-NBOMe is a Schedule I controlled substance.[25]
- New Zealand: 25B-NBOMe is a Schedule 2 controlled substance in New Zealand.[26]
- Sweden: 25B-NBOMe is classed as Schedule I.[27]
- Switzerland: 25B-NBOMe is a controlled substance specifically named under Verzeichnis D.[28]
- Turkey: 25B-NBOMe is a classed as drug and is illegal to possess, produce, supply, or import.[29] [30]
- United Kingdom: 25B-NBOMe is a Class A drug in the United Kingdom as a result of the N-benzylphenethylamine catch-all clause.[31]
- United States: On Nov 15, 2013, the DEA added 25B-NBOMe to Schedule I using their emergency scheduling powers, making it "temporarily" in Schedule I for 2 years.[32]
See also
External links
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Erowid NBOMe (Other or Unknown NBOMe-Compound) Vault : Fatalities / Deaths
- ↑ 2.0 2.1 Heim, R. (2004). "Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2 -Methoxybenzyl-Partialstruktur: Entwicklung eines neuen Struktur-Wirkungskonzepts" (in German). doi:10.17169/refubium-16193.
- ↑ 3.0 3.1 3.2 Hansen, M., Phonekeo, K., Paine, J. S., Leth-Petersen, S., Begtrup, M., Bräuner-Osborne, H., Kristensen, J. L. (19 March 2014). "Synthesis and Structure–Activity Relationships of N -Benzyl Phenethylamines as 5-HT 2A/2C Agonists". ACS Chemical Neuroscience. 5 (3): 243–249. doi:10.1021/cn400216u. ISSN 1948-7193.
- ↑ Erowid Bromo-Dragonfly Vault : Dosage
- ↑ Silva, Maria Elena (2009). "Theoretical study of the interaction of agonists with the 5-HT2A receptor". doi:10.5283/EPUB.12119.
- ↑ Silva, M. E., Heim, R., Strasser, A., Elz, S., Dove, S. (January 2011). "Theoretical studies on the interaction of partial agonists with the 5-HT2A receptor". Journal of Computer-Aided Molecular Design. 25 (1): 51–66. doi:10.1007/s10822-010-9400-2. ISSN 0920-654X.
- ↑ Designer Drug Identified As Cause Of Plano Teen’s Death
- ↑ Preclinical Safety Assessment of the 5-HT2A Receptor Agonist PET Radioligand [11C]Cimbi-36 | https://bitnest.netfirms.com/external.php?id=%257DbxUgX%255DCY%2504%2505wzx%2519%2505VYL%2502RI%257E%2560d
- ↑ Erowid 25I-NBOMe (2C-I-NBOMe) Vault : Fatalities / Deaths
- ↑ Erowid 2C-C-NBOMe (25C-NBOMe) Vault : Fatalities / Deaths
- ↑ Lützen, E., Holtkamp, M., Stamme, I., Schmid, R., Sperling, M., Pütz, M., Karst, U. (April 2020). "Multimodal imaging of hallucinogens 25C‐ and 25I‐NBOMe on blotter papers". Drug Testing and Analysis. 12 (4): 465–471. doi:10.1002/dta.2751. ISSN 1942-7603.
- ↑ Marchi, N. C., Scherer, J. N., Fara, L. S., Remy, L., Ornel, R., Reis, M., Zamboni, A., Paim, M., Fiorentin, T. R., Wayhs, C. A. Y., Von Diemen, L., Pechansky, F., Kessler, F. H. P., Limberger, R. P. (1 March 2019). "Clinical and Toxicological Profile of NBOMes: A Systematic Review". Psychosomatics. 60 (2): 129–138. doi:10.1016/j.psym.2018.11.002. ISSN 0033-3182.
- ↑ Yoon, K. S., Yun, J., Kim, Y.-H., Shin, J., Kim, S. J., Seo, J.-W., Hyun, S.-A., Suh, S. K., Cha, H. J. (1 April 2019). "2-(2,5-Dimethoxy-4-methylphenyl)-N-(2-methoxybenzyl)ethanamine (25D-NBOMe) and N-(2-methoxybenzyl)-2,5-dimethoxy-4-chlorophenethylamine (25C-NBOMe) induce adverse cardiac effects in vitro and in vivo". Toxicology Letters. 304: 50–57. doi:10.1016/j.toxlet.2019.01.004. ISSN 0378-4274.
- ↑ https://psychonautwiki.org/wiki/File:Nbome_death_news_i2013e0190_disp.jpg
- ↑ https://psychonautwiki.org/wiki/File:Nbome_death_news_i2013e0191_disp.jpg
- ↑ https://www.ris.bka.gv.at/Dokumente/BgblAuth/BGBLA_2019_II_167/BGBLA_2019_II_167.pdfsig
- ↑ http://portal.anvisa.gov.br/documents/10181/3115436/%281%29RDC_130_2016_.pdf/fc7ea407-3ff5-4fc1-bcfe-2f37504d28b7
- ↑ Branch, L. S. (2022), Consolidated federal laws of Canada, Controlled Drugs and Substances Act
- ↑ 关于印发《非药用类麻醉药品和精神药品列管办法》的通知 | http://www.sfda.gov.cn/WS01/CL0056/130753.html
- ↑ "Achtundzwanzigste Verordnung zur Änderung betäubungsmittelrechtlicher Vorschriften" (PDF) (in German). Bundesanzeiger Verlag. Retrieved December 11, 2019.
- ↑ "Anlage I BtMG" (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 11, 2019.
- ↑ "§ 29 BtMG" (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 11, 2019.
- ↑ Tabella 1 Stupefacenti dello Stato Italiano |http://www.salute.gov.it/imgs/C_17_pagineAree_3729_listaFile_itemName_0_file.pdf
- ↑ "新たに4物質を麻薬に指定し、規制の強化を図ります" (in Japanese). 厚生労働省 [Ministry of Health, Labour and Welfare (MHLW)]. Retrieved May 2, 2022.
- ↑ Zaudējis spēku - Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem
- ↑ Misuse of Drugs Act 1975 No 116 (as at 07 December 2021), Public Act – New Zealand Legislation
- ↑ Läkemedelsverkets författningssamling (PDF)
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
- ↑ Başbakanlık Mevzuatı Geliştirme ve Yayın Genel Müdürlüğü
- ↑ https://resmigazete.gov.tr/eskiler/2014/01/20140125-3-1.pdf
- ↑ Misuse of Drugs Act 1971 (S.I. 2014/1106), London: The Stationery Office Limited, 2014, retrieved 5 July 2017
- ↑ http://www.justice.gov/dea/divisions/hq/2013/hq111513.shtml