Talk:Dissociatives
Dissociatives (also known as dissociative anesthetics) refer to a class of hallucinogen which distort sensory perceptions (mainly of sight and sound) to produce feelings of disconnection, detachment, and dissociation from the environment and self. This is done by reducing or blocking signals to the conscious mind from other parts of the brain.[1]
Although many classes of psychoactive substances are capable of such action, dissociatives are unique in that they do so in such a way that they produce hallucinogenic effects, which generally include sensory deprivation, dissociation, hallucinations, and dream-like states or trances.[2] Some dissociatives, which are non-selective in action and affect the dopamine[3] and/or opioid[4] systems, may also be capable of inducing euphoria.
Mechanism of action
NMDA receptors within the brain exist to allow for the transfer of electrical signals between neurons in the brain and in the spinal column. For electrical signals to pass, the NMDA receptor must be open. To remain open, the neurotransmitters known as glutamate and glycine must bind to the NMDA receptor. An NMDA receptor that has glycine and glutamate bound to it and has an open ion channel is called "activated."
Dissociatives are classed as NMDA receptor antagonists. This means they bind to the receptor, but do not activate it and block other neurotransmitters from doing so. The result is a dose dependent decrease in the passing of electrical signals across the brain and an overall disconnection of neurons. This leads onto states of disconnection between conscious parts of the brain and its sensory organs as well as out-of-body experiences and accompanying hallucinations.
Subjective effects
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Visual effects 
-
Suppressions
-
Distortions
-
Geometry
In comparison to other classes of hallucinogen, this effect is significantly less complex and intricate with a limited range of effects. It does not extend beyond level 4 and is variable within most of its variations but is exclusively simplistic in complexity, unstructured in organization, dimly lit in lighting, slow in movement and immersive in depth.
-
Hallucinatory states
- Perspective alterations
- Autonomous entities
- Scenarios and plots
- Settings, sceneries, and landscapes
- Shadow people
External hallucinations
In comparison to other classes of hallucinogen, this effect can occur at heavy dosages, but is extremely infrequent in comparison to the same effect found within deliriants.It can be comprehensively described through its variations as delirious in believability, autonomous in controllability and solid in appearance.
The most common theme for this effect to follow is one of experiencing and talking to friends around oneself when they are not actually present.
Internal hallucinations
In comparison to other classes of hallucinogen, this effect can occur at heavy dosages but is considerably less common than the same effect found within psychedelics and deliriants.It can be comprehensively described through its variations as delirious in believability, fixed in style, equal in new experiences and memory replays in content, autonomous in controllability and solid in appearance.
Disconnective effects 
Cognitive effects 
-
- Amnesia
- Conceptual thinking
- Déjà vu
- Depersonalization
- Derealization
- Dream potentiation
- Increased music appreciation
- Information processing suppression
- Introspection
- Memory suppression
- Personal bias suppression
- Subconscious communication
- Thought connectivity
- Thought deceleration
- Thought loops
- Time distortion
- Unity and interconnectedness
Physical effects 
Auditory effects 
Multisensory effects 
Pharmacological classes
Morphinans
-
- Dextromethorphan (DXM)
- Dextrorphan (DXO)
Conantokins Peptides
-
- Conantokin-G (Con-G)
- Conantokin-T (Con-T)
- Conantokin-R (Con-R)
- Conantokin-L (Con-L)
- Conantokin-P (Con-Pr1, Con-Pr2 and Con-Pr3)
- Conantokin-Rl-A(Con-Rl-A)
- Conantokin-Br(Con-Br)
Toxicity and harm potential
 |
This toxicity and harm potential section is a stub. As a result, it may contain incomplete or even dangerously wrong information! You can help by expanding upon or correcting it. |
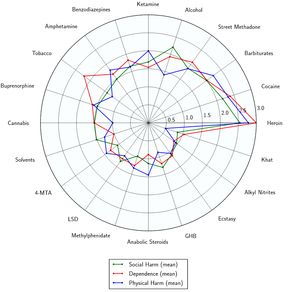
Ketamine
Fatal ketamine overdoses are particularly rare, but not completely unheard of. However, the exact toxic dosage is unknown.
The first large-scale, longitudinal study of ketamine users found that frequent ketamine users (at least 4 days/week, averaging 20 days/month) had increased depression and impaired memory by several measures, including verbal, short-term memory and visual memory. However, infrequent (1–4 days/month, averaging 3.25 days/month) ketamine users and former ketamine users were not found to differ from controls in memory, attention and psychological well-being tests. This suggests the infrequent use of ketamine does not cause cognitive deficits and that any deficits that might occur may be reversible when ketamine use is discontinued. However, abstinent, frequent, and infrequent users all scored higher than controls on a test of delusional symptoms.[6]
Short-term exposure of cultures of GABAergic neurons to ketamine at high concentrations led to a significant loss of differentiated cells in one study, and non-cell death-inducing concentrations of ketamine (10 μg/ml) may still initiate long-term alterations of dendritic arbor in differentiated neurons. The same study also demonstrated chronic (>24 h) administration of ketamine at concentrations as low as 0.01 μg/ml can interfere with the maintenance of dendritic arbor architecture. These results raise the possibility that chronic exposure to low, subanesthetic concentrations of ketamine, while not affecting cell survival, could still impair neuronal morphology and thus might lead to dysfunctions of neural networks.[7] [8]
More recent studies of ketamine-induced neurotoxicity have focused on primates in an attempt to use a more accurate model than rodents. One such study administered daily ketamine doses consistent with typical recreational doses (1 mg/kg IV) to adolescent cynomolgus monkeys for varying periods of time. Decreased locomotor activity and indicators of increased cell death in the prefrontal cortex were detected in monkeys given daily injections for six months, but not those given daily injections for one month.[9]
Studies have shown that the serotonin systems affected by such serotonergic drugs are linked to the NMDA/glutamate systems.[10] Tests on rats indicate that 5-HT agonists like LSD and psilocybin can prevent neurotoxicity due to NMDA receptor antagonists.[11]
Urinary tract effects
In general, dissociatives are considered to have harmful effects on the urinary tract of humans when used heavily and/or for extended periods of time for reasons that have yet to be fully understood.[citation needed]
For example, according to a recent systematic review, 110 documented reports of irritative urinary tract symptoms from ketamine dependence exist.[12] Urinary tract symptoms have been collectively referred to as "ketamine-induced ulcerative cystitis" or "ketamine-induced vesicopathy" and they include urge incontinence, decreased bladder compliance, decreased bladder volume and painful haematuria (blood in urine).
The time of onset of lower urinary tract symptoms varies depending, in part, on the severity and chronicity of ketamine use; however, it is unclear whether the severity and chronicity of ketamine use corresponds linearly to the presentation of these symptoms. All reported cases where the user consumed greater than 5 grams per day reported symptoms of the lower urinary tract.[13]
It is strongly recommended that one use harm reduction practices when using this drug.
Tolerance and addiction potential
Although there is a significant amount of variation within the NMDA receptor antagonist family, their chronic use is typically considered moderately addictive with a high potential for abuse and are capable of causing psychological dependence among certain users. When addiction has developed, cravings and withdrawal effects may occur if a person suddenly stops their usage.
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- Stimulants - Both stimulants and dissociatives carry the risk of adverse psychological reactions like anxiety, mania, delusions and psychosis and these risks are exacerbated when the two substances are combined.
- Depressants - Because both depress the respiratory system, this combination can result in an increased risk of suddenly falling unconscious, vomiting and choking to death from the resulting suffocation. If nausea or vomiting occurs, users should attempt to fall asleep in the recovery position or have a friend move them into it.
See also
References
- ↑ PCP-induced alterations in cerebral glucose utilization in rat brain: blockade by metaphit, a PCP-receptor-acylating agent.| http://www.ncbi.nlm.nih.gov/pubmed/2850626
- ↑ Snyder, Solomon H. (1980). "Phencyclidine". Nature 285 (5764): 355–6. | http://www.nature.com/nature/journal/v285/n5764/abs/285355a0.html
- ↑ Giannini, A. James; Nageotte, Catherine; Loiselle, Robert H.; Malone, Donald A.; Price, William A. (1984). "Comparison of Chlorpromazine, Haloperidol and Pimozide in the Treatment of Phencyclidine Psychosis: Da-2 Receptor Specificity". Clinical Toxicology 22 (6): 573–9. (PubMed.gov / NCBI) | http://www.ncbi.nlm.nih.gov/pubmed/6725621
- ↑ Giannini, A. James; Underwood, Ned A.; Condon, Maggie (2000). "Acute Ketamine Intoxication Treated by Haloperidol". American Journal of Therapeutics 7 (6): 389–91. (PubMed.gov / NCBI) | http://www.ncbi.nlm.nih.gov/pubmed/11304647
- ↑ Development of a rational scale to assess the harm of drugs of potential misuse (ScienceDirect) | http://www.sciencedirect.com/science/article/pii/S0140673607604644
- ↑ "Addiction Users Study : Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study - http://onlinelibrary.wiley.com/doi/10.1111/j.1360-0443.2009.02761.x/abstract
- ↑ Low concentrations of ketamine initiate dendritic atrophy of differentiated GABAergic neurons in culture (ScienceDirect) | http://www.sciencedirect.com/science/article/pii/S0300483X07001138
- ↑ Neuroprotective NMDA antagonists: the controversy over their potential for adverse effects on cortical neuronal morphology (PubMed.gov / NCBI) | http://www.ncbi.nlm.nih.gov/pubmed/7976530
- ↑ Chronic ketamine exposure induces permanent impairment of brain functions in adolescent cynomolgus monkeys | http://onlinelibrary.wiley.com/doi/10.1111/adb.12004/abstract
- ↑ author=Arvanov V, Liang X, Russo A, Wang R |title=LSD and DOB: interaction with 5-HT2A receptors to inhibit NMDA receptor-mediated transmission in the rat prefrontal cortex | http://onlinelibrary.wiley.com/doi/10.1046/j.1460-9568.1999.00726.x/abstract
- ↑ Farber N, Hanslick J, Kirby C, McWilliams L, Olney J | Serotonergic agents that activate 5HT2A receptors prevent NMDA antagonist neurotoxicity | http://www.nature.com/npp/journal/v18/n1/full/1395108a.html
- ↑ Ketamine-induced vesicopathy: a literature review | http://onlinelibrary.wiley.com/doi/10.1111/j.1742-1241.2010.02502.x/abstract
- ↑ Ketamine use: a review | http://onlinelibrary.wiley.com/doi/10.1111/j.1360-0443.2011.03576.x/abstract