Talk:Clobenzorex
This page has not been fully approved by the PsychonautWiki administrators. It may contain incorrect information, particularly with respect to dosage, duration, subjective effects, toxicity and other risks. It may also not meet PW style and grammar standards. |
| Summary sheet: Clobenzorex |
| Clobenzorex | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||||
| Common names | Clobenzorex, Greenies, Asenlix, Giranza, Itravil, Obeclox[2] | ||||||||||||||||||||||||||||||||||
| Substitutive name | N-(2-Chlorobenzyl)amphetamine | ||||||||||||||||||||||||||||||||||
| Systematic name | N-(2-Chlorobenzyl)-1-phenylpropan-2-amine | ||||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||||
| Psychoactive class | Stimulant | ||||||||||||||||||||||||||||||||||
| Chemical class | Amphetamine / NBx | ||||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
Clobenzorex (also known as CLX, greenies, and the trade names Asenlix, Giranza, Itravil and Obeclox, among others) is a lesser-known stimulant substance of the amphetamine class. It is a prodrug for dextroamphetamine (d-amphetamine) that is widely used in the management of nonresponder obesity cases.[4]. Like amphetamine, clobenzorex produces its effects by promoting the release of neurotransmitters dopamine and norepinephrine in the brain.
In commercial production, clobenzorex is supplied as the hydrochloride salt in green-tinted capsules. The drug gained use as a prescription anorectic in the 1970s; however, frequent instances of abuse were eventually observed, which led to the withdrawal of clobenzorex from the market in the US and certain other countries in 2000.[5] Although, there is evidence of its availability along the USA-Mexico border.[citation needed]
Subjective effects include stimulation, focus enhancement, motivation enhancement, increased libido, appetite suppression and euphoria. It is exclusively taken orally since its a prodrug, This means that insufflation, smoking, injection, etc. do not provide faster absorption or onset. It is generally compared to lisdexamfetamine in duration and efficacy, with half the potency by weight. Some users also report it produces a softer comedown relative to amphetamine.
However, it is highly advised to use harm reduction practices if using this substance.
History and culture
This History and culture section is a stub. As a result, it may contain incomplete or wrong information. You can help by expanding it. |
Clobenzorex was first manufactured by Hoechst Marion Roussel (Aventis) as a sympathomimetic amine with low addictive activity in the treatment of obesity in patients who did not respond to diet and excercise.[6]
The drug is legally distributed in Mexico to treat obesity[7]. In Mexico, it is one of the five main anorectic drugs used to treat obesity.[citation needed]
In the United States of America, clobenzorex tablets (among other varieties of stimulants, such as amphetamine) were used by athletes who ingested the drug to reduce fatigue, increase attention, and improve reaction times during athletic activities. The green-tinted Asenlix capsules (generic forms can be seen as half light green, half dark green capsules marked "IFA") were known as "greenies" among US baseball players, a slang term that in current use has expanded to generically refer to any amphetamine-class stimulant.[8]
In Brazil, it has been identified in pills seized from truck drivers who consume them to remain awake and reduce fatigue when driving long distances.[citation needed]
Chemistry
Clobenzorex is a synthetic molecule of the substituted amphetamine class. Molecules of the amphetamine class contain a phenethylamine core featuring a phenyl ring bound to an amino (NH2) group through an ethyl chain with an additional methyl substitution at Rα (i.e., amphetamines are alpha-methylated phenethylamines). Clobenzorex contains a benzyl group bound to the terminal amine RN of the amphetamine core, a substitution it shares with benzphetamine. Additionally, it contains a chloride atom at the ortho position of the N-benzyl group.
Clobenzorex is also classified as an NBx compound. (?)
Pharmacology
Clobenzorex was developed with the goal of providing superior anorexegenic effects to other amphetamines as well as less cardiovascular side effects to other amphetamines. The attachment of the functional group o-chlorobenzyl amino reduces the relative percentage of d-amphetamine that is enzymatically cleaved from the parent compound. Because no free d-amphetamine is present in clobenzorex capsules, d-amphetamine does not become available through mechanical manipulation, such as crushing or simple extraction. There is, therefore, no way to speed up absorption via alternate routes of administration, such as via insufflation, vaporization, or injection, making the drug theoretically less abusable.
Pharmacodynamics
Dextroamphetamine is a full agonist of the trace amine-associated receptor 1 (TAAR1), which is a key regulator of common and trace brain monoamines such as dopamine, serotonin and noradrenaline.[9][10][11] The agonism of this set of receptors results in the release of increased concentrations of dopamine, serotonin and noradrenaline in the synaptic cleft. This leads to cognitive and physical stimulation within the user.
Clobenzorex and other N-alkylamphetamines exert their own unique effects, notably:
- Suppression of appetite through activation of α4 and β1 adrenergic receptors in the hypothalamus[12]
- Dose-dependent vasorelaxation of the left aorta via the NO/cGMP/PKG/Ca²⁺-activated K⁺ channel pathway[13]
- Increased breakdown of FFAs, glucose, and cHDL in subcutaneous adipose tissue[14]
- Decreased 11β-HSD1 hydrogenease activity in vitro and decreased 11β-HSD1 transcription and serum cortisol ex vivo in mesenteric adipose tissue[15]
- Activation of the TrKβ receptor[16]
Pharmacokinetics
As a prodrug, clobenzorex is inactive in the form administered and requires first-pass metabolism to “activate”. CYP1A2, CYP2B6, and CYP2C19 convert the majority into 4-hydroxyclobenzorex; a small amount is enzymatically processed by CYP3A4 and CYP2B6 as d-amphetamine.[17] Both resultant forms are further metabolized into their respective inactive conjugates.
Clobenzorex can be detected in urine, which can cause false positives for workplace drug screening.[citation needed] It is one of many drugs that can cause false positives for amphetamine urine drug screening.[citation needed] It may be differentiated from amphetamine use through testing for 4-hydroxyclobenzorex[18] or enantiomeric analysis.[citation needed]
Conversion rate
5.9–15.4% of the weight of clobenzorex hydrochloride (the usual prescribed form) is dextroamphetamine: 30 mg clobenzorex hydrochloride is equivalent to 1.77–4.62 mg of dextroamphetamine.[19]
The subjective experience will differ due to the slower, more steady onset of active substance in the prodrug. An equivalent dose of dextroamphetamine will have a higher peak plasma concentration and shorter duration.
10.1–40.4% of the weight of clobenzorex hydrochloride is 4-hydroxyclobenzorex: 30 mg clobenzorex hydrochloride is equivalent to 3.03–12.12 mg of 4-hydroxyclobenzorex.[20]
Effective dose
The ED50 (the dose that produces a “quantal effect” for 50% of the population base) of clobenzorex in mice has been found to be 6.6 mg/kg (0.3 mg/kg for d-amphetamine).[21]
Subjective effects
In comparison to other substituted amphetamines, Clobenzorex is reported to be relatively free of side effects such as nausea, high blood pressure, anxiety and an uncomfortable offset ("comedown"). While the subjective effects are almost identical to that of lisdexamfetamine, clobenzorex is half the potency by weight and a few hours longer in duration, albeit inconsistent in part of its lower, invariable yield of its active conjugate. However, at higher doses, it typically loses its productivity and focus-enhancing effects and begins to take on a recreational character due to the distracting euphoria that it can produce. The parent compound also carries its own set of mechanisms of action not found in other amphetamines.
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Stimulation - Clobenzorex is reported to be very energetic and stimulating in a manner similar to lisdexamfetamine. It can encourage physical activities such as dancing, socializing, running, or cleaning. The particular style of stimulation which clobenzorex produces can be described as forced. This means that, at higher dosages, it becomes difficult or impossible to keep still as jaw clenching, involuntary bodily shakes and vibrations become present, resulting in extreme shaking of the entire body, unsteadiness of the hands, and a general loss of fine motor control. This effect is replaced with mild fatigue and general exhaustion during the offset of the experience.
- Spontaneous bodily sensations - The "body high" of clobenzorex can be described as a moderate euphoric tingling sensation that encompasses the entire body. This sensation maintains a consistent presence that steadily rises with the onset and hits its limit once the peak has been reached.
- Physical euphoria
- Abnormal heartbeat[22]
- Increased heart rate
- Appetite suppression - This effect is more pronounced compared to amphetamine, sometimes causing people to not eat for the entire duration of action. Clobenzorex is commonly prescribed to treat obesity due to its strong appetite suppressing effect.[23]
- Bronchodilation
- Muscle tension
- Dehydration
- Dry mouth
- Frequent urination
- Increased bodily temperature
- Increased perspiration
- Nausea - This effect usually only occurs at heavy doses.
- Pupil dilation - This effect is more prominent on the offset of the experience and usually only occurs at common to heavy doses.
- Stamina enhancement
- Teeth grinding - This component can be considered to be less intense when compared with that of MDMA.
- Temporary erectile dysfunction
- Vasoconstriction
- Vasodilation - This effect is unique to the N-alkylamphetamines. ?.[24]
Visual effects 
-
The visual effects of clobenzorex are usually less consistent and only mildly noticeable at higher dosages. They are somewhat comparable to the visual effects of deliriants and occur more readily in darker areas.
Enhancements
- Visual acuity enhancement
- Double vision - Amphetamines can cause double vision at high doses.
Distortions
- Drifting (breathing and morphing) - This effect is usually subtle and only occurs at higher doses, after long periods of being awake, or when combined with cannabis. Commonly this consists of level 1-2 drifting.
- Brightness alteration - Clobenzorex can make spaces seem brighter as a result of its pupil dilating effects.
Hallucinatory states
- Transformations - This effect occurs very rarely, and typically only when the user has taken high doses, is coming down, or has been awake for unusually long periods. They are usually very mild when they do occur.
Cognitive effects 
-
Clobenzorex shares most of its cognitive effects with other amphetamines, although it is less forceful in its come up due to the slower metabolism. It produces a variety of cognitive enhancements associated with stimulants. However, during the latter part of the duration, these cognitive enhancements may compete with or be nullified by the accumulated dopamine depletion and its effects.
The most prominent of these cognitive effects generally include:
- Analysis enhancement
- Anxiety - This effect occurs more frequently on the offset phase of the experience.
- Creativity enhancement
- Compulsive redosing - Due to the slow come up of clobenzorex, the full effects may not be felt for up to three hours after consumption, causing some to redose during the come up. Compulsive redosing is more common if heavy doses are taken.
- Ego inflation
- Emotion suppression
- Focus enhancement
- Irritability
- Immersion enhancement
- Increased music appreciation
- Memory enhancement
- Motivation enhancement
- Increased libido or Decreased libido
- Novelty enhancement
- Time distortion - This can be described as the experience of time speeding up and passing much quicker than it usually would when sober.
- Thought acceleration
- Thought organization
- Wakefulness
- Cognitive euphoria
Auditory effects 
-
- Enhancements
- Hallucinations - Use of clobenzorex and other amphetamines, at a strong or heavy dose, can occasionally cause mild auditory hallucinations. These hallucinations most commonly occur amongst a source of white noise, such as a fan, and typically consist of quiet phantom music or voices. Clobenzorex may also cause auditory hallucinations in the form of stimulant psychosis.
After effects 
- The effects which occur during the offset of a stimulant experience generally feel negative and uncomfortable in comparison to the effects which occurred during its peak. This is often referred to as a "comedown" and occurs because of neurotransmitter depletion. The comedown produced by clobenzorex is often reported to be much less intense than its metabolite, dextroamphetamine, due to its ?. Its effects commonly include: Making sure to eat well and to hydrate are recommended to decrease the severity of comedown effects. Using mild sedatives is also a common strategy for stimulant comedowns.
Experience reports
There are currently 0 experience reports which describe the effects of this substance in our experience index.
Toxicity and harm potential
 |
This toxicity and harm potential section is a stub. As a result, it may contain incomplete or even dangerously wrong information! You can help by expanding upon or correcting it. |

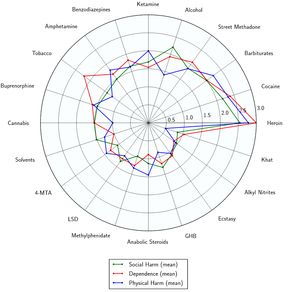
As of March 2014, there is no evidence that amphetamine is directly neurotoxic in humans.[27] However, high-dose amphetamine can cause indirect neurotoxicity as a result of increased oxidative stress from reactive oxygen species and autoxidation of dopamine.[28][29][30]
In rodents and primates, sufficiently high doses of amphetamine causes damage to dopamine neurons, characterized as reduced transporter and receptor function.[31] Animal models of neurotoxicity from high-dose amphetamine exposure indicate that the occurrence of hyperpyrexia (i.e., core body temperature ≥ 40 °C) is necessary for the development of amphetamine-induced neurotoxicity. [32]
Melatonin has been shown to prevent (if used 30min+ before dosing) and reverse amphetamine induced neurotoxicity of TH-pSer40 and calpastatin levels in the Substantia Nigra of rats.[33][34]
It is strongly recommended that one use harm reduction practices when using this substance.
Lethal dosage
The LD50 (the dosage required to kill 50% of the test subjects) of clobenzorex in mice has been found to be 103 mg per kilogram.[35] No formal studies in humans have been carried out and the exact toxic dosage is unknown.
Dependence and abuse potential
Amphetamine has high abuse potential and can cause psychological dependence with chronic use.
When dependence has developed, cravings and withdrawal effects may occur if use is suddenly discontinued.[45][46] Withdrawal symptoms include paranoia, depression, dream potentiation, anxiety, itching, mood swings, irritability, fatigue, insomnia, an intense craving for more amphetamine or other stimulants.
Addiction is a serious risk with heavy recreational amphetamine use but is unlikely to arise from typical long-term medical use at therapeutic doses. Clobenzorex has been posited to have less potential for abuse and addiction than other pharmaceutical amphetamines due to the slower onset and ? metabolism, which ? and consequent dopamine release. Caution is nonetheless advised, as with other drugs in the amphetamine class.
Tolerance to many of the effects of amphetamine develops with prolonged and repeated use. This results in the user having to administer increasingly large doses to achieve the same effects. Upon single administration, it takes about 3 - 7 days for the tolerance to be reduced to half and 1 - 2 weeks to be back at baseline (in the absence of further consumption).
Likewise to amphetamine, clobenzorex exhibits cross-tolerance with all dopaminergic stimulants, meaning that after consumption it will have a reduced effect.
Psychosis
Severe amphetamine overdose can result in a stimulant psychosis that may present with a variety of symptoms (e.g., paranoia, hallucinations, delusions).[36] A review on treatment for amphetamine abuse-induced psychosis states that about 5–15% of users fail to recover completely.[36][37] The same review asserts that antipsychotic medications effectively resolve the symptoms of acute amphetamine psychosis.[36] Psychosis very rarely arises from therapeutic use.[38]
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- Alcohol - Drinking alcohol on stimulants is considered risky because it reduces the sedative effects of the alcohol that the body uses to gauge drunkenness. This often leads to excessive drinking with greatly reduced inhibitions, increasing the risk of liver damage and increased dehydration. The effects of stimulants will also allow one to drink past a point where they might normally pass out, increasing the risk. If you do decide to do this then you should set a limit of how much you will drink each hour and stick to it, bearing in mind that you will feel the alcohol and the stimulant less.
- GHB/GBL - Stimulants increase respiration rate allowing a higher dose of sedatives. If the stimulant wears off first then the depressant effects of the GHB/GBL may overcome the user and cause respiratory arrest.
- Opioids - Stimulants increase respiration rate allowing a higher dose of opiates. If the stimulant wears off first then the opiate may overcome the patient and cause respiratory arrest.
- Cocaine - The rewarding effects of cocaine are mediated by DAT inhibition, and an increase of exocytosis of dopamine through the cell membrane. Amphetamine reverses the direction of DAT and the direction vesicular transports within the cell by a pH mediated mechanism of displacement, thus excludes the regular mechanism of dopamine release through means of exocytosis because the effects Na+/K+ ATPase are inhibited. You will find cardiac effects with the combination of cocaine and amphetamine due to a SERT mediated mechanism from the subsequent activation of 5-HT2B, which is an effect of serotonin-related valvulopathy. Amphetamines generally cause hypertension in models of abuse, and this combination can increase the chances of syncope due to turbulent blood flow during valve operation. The rewarding mechanisms of cocaine are reversed by administration of amphetamine.[39][40]
- Cannabis - Stimulants increase anxiety levels and the risk of thought loops and paranoia which can lead to negative experiences.
- Caffeine - This combination of stimulants is generally considered unnecessary and may increase strain on the heart, as well as potentially causing anxiety and physical discomfort.
- Tramadol - Tramadol and stimulants both increase the risk of seizures.
- DXM - Both substances raise heart rate, in extreme cases, panic attacks caused by these substances have led to more serious heart issues.
- Ketamine - Combining amphetamine and ketamine may result in psychoses that resemble schizophrenia, but not worse than the psychoses produced by either substance alone, but this is debatable. This is due to amphetamines ability to attenuated the disruption of working memory caused by ketamine. Amphetamine alone may result in grandiosity, paranoia, or somatic delusions with little to no effect on negative symptoms. Ketamine, however, will result in thought disorders, disruption of executive functioning, and delusions due to a modification of conception. These mechanisms are due to an increase of dopaminergic activity in the mesolimbic pathway caused by amphetamine due to its pharmacology effecting dopamine, and due to a disruption of dopaminergic functioning in the mesocortical pathways via NMDA antagonism effects of ketamine. Combining the two, you may expect mainly thought disorder along with positive symptoms.[41]
- PCP - Increases risk of tachycardia, hypertension, and manic states.
- Methoxetamine - Increases risk of tachycardia, hypertension, and manic states.
- Psychedelics (e.g. LSD, mescaline, psilocybin) - Increases risk of anxiety, paranoia, and thought loops.
- 25x-NBOMe - Amphetamines and NBOMes both provide considerable stimulation that when combined they can result in tachycardia, hypertension, vasoconstriction and, in extreme cases, heart failure. The anxiogenic and focusing effects of stimulants are also not good in combination with psychedelics as they can lead to unpleasant thought loops. NBOMes are known to cause seizures and stimulants can increase this risk.
- 2C-T-x - Suspected of mild MAOI properties. May increase the risk of hypertensive crisis.
- 5-MeO-xxT - Suspected of mild MAOI properties. May increase the risk of hypertensive crisis.
- DOx
- aMT - aMT has MAOI properties which may interact unfavorably with amphetamines.
- MAOIs - MAO-B inhibitors can increase the potency and duration of phenethylamines unpredictably. MAO-A inhibitors with amphetamine can lead to hypertensive crises.
Legal status
Clobenzorex is predominantly legal, but in a few countries it is controlled.
- Brazil: Clobenzorex is listed as Class F2 (Prohibited psychotropics).[42]
- Canada: Clobenzorex is not specifically listed in the CDSA, however due to structural similarities with benzphetamine, it is a Schedule I under item 19(17).[43]
- United Kingdom: Clobenzorex is a Class B controlled drug.[44]
- United States: Clobenzorex is not scheduled and is unaffected by the Federal Analogue Act as a derivative of benzphetamine.[45]
- Importation for personal use is lawful provided that is for use to treat a condition with no approved medications, unlawful marketing is not occurring in the U.S, not deemed hazardous to health for the treating the condition, and is verified as a continuation of a treatment plan that began in a foreign country.[46]
The use of clobenzorex is banned by the World Anti-Doping Agency for use during sports competitions.[47]
See also
External links
Literature
- ?
References
- ↑ Onakpoya, I. J., Heneghan, C. J., & Aronson, J. K. (2013). "Comparison on the pharmacokinetics and weight reduction of clobenzorex slow release and immediate release formulations in obese patients". Pharmacology & Pharmacy. 4 (2): 218–221. doi:10.4236/pp.2013.42030.
- ↑ https://www.medicamentosplm.com/Home/Medicamentos_Sustancia/clobenzorex/292
- ↑ [citation needed]
- ↑ Cody JT (2005). "Amphetamines: Methods of forensic analysis.". In Smith F, Athanaselis SS. Handbook of Forensic Drug Analysis. Elsevier. pp. 357–451 (430). ISBN 978-0-08-047289-8.
Amphetamine produced from the metabolism of clobenzorex has been shown to be the d-enantiomer only ...
- ↑ Onakpoya, I. J., Heneghan, C. J., & Aronson, J. K. (2013). "Comparison on the pharmacokinetics and weight reduction of clobenzorex slow release and immediate release formulations in obese patients". Pharmacology & Pharmacy. 4 (2): 218–221. doi:10.4236/pp.2013.42030.
- ↑ Argüelles-Tello, F., Carrasco-Portugal, M., Carrasco-Portugal, N., Aguilar-Carrasco, J., Patiño-Camacho, S., Valle, C., Reyes-Garcia, G., & Flores-Murrieta, F. (2013). "Comparison on the pharmacokinetics and weight reduction of clobenzorex slow release and immediate release formulations in obese patients". Pharmacology & Pharmacy. 4 (2): 218–221. doi:10.4236/pp.2013.42030.
- ↑ Young, R., Darmani, N. A., Elder, E. L., Dumas, D., & Glennon, R. A. (1997). "Clobenzorex: Evidence for amphetamine-like behavioral actions". Pharmacology, Biochemistry, and Behavior. 56 (2): 311–316. doi:10.1016/s0091-3057(96)00329-2.
- ↑ https://www.chemeurope.com/en/encyclopedia/Clobenzorex.html#_note-0
- ↑ Miller, G. M. (2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". Journal of Neurochemistry. 116 (2): 164–176. doi:10.1111/j.1471-4159.2010.07109.x.
- ↑ Drug banks amphetamine targets
- ↑ TA1 receptor |http://www.iuphar-db.org/DATABASE/ObjectDisplayForward?objectId=364
- ↑ Argüelles-Tello, F., Carrasco-Portugal, M., Carrasco-Portugal, N., Aguilar-Carrasco, J., Patiño-Camacho, S., Valle, C., Reyes-Garcia, G., & Flores-Murrieta, F. (2013). "Comparison on the pharmacokinetics and weight reduction of clobenzorex slow release and immediate release formulations in obese patients". Pharmacology & Pharmacy. 4 (2): 218–221. doi:10.4236/pp.2013.42030.
- ↑ Lozano-Cuenca, J., González-Hernández, A., López-Canales, O. A., Villagrana-Zesati, J. R., Rodríguez-Choreño, J. D., Morín-Zaragoza, R., Castillo-Henkel, E. F., & López-Canales, J. S. (2017). "Possible mechanisms involved in the vasorelaxant effect produced by clobenzorex in aortic segments of rats". Brazilian Journal of Medical and Biological Research. 50 (9). doi:10.1590/1414-431X20175765.
- ↑ Gabriela, C.-M., Adrian, H. H., Ángel, M.-G., Raúl, M.-Z., Rafael, G.-D., & Eleazar, L.-P. (2017). "Possible peripheral lipolytic effect of clobenzorex hydrochloride in patients with obesity". Medico Research Chronicles. 4 (3): 252–265. ISSN 2394-3971.
- ↑ C. Pérez, L. C., Padilla-Martínez, I. I., Cruz, A., Basurto, J. C., García, Á. M., H. Zavala, A. A., López, M. G., & R. Hernández, M. C. (2020). "Design, synthesis, molecular docking and in vitro evaluation of benzothiazole derivatives as 11β-hydroxysteroid dehydrogenase type 1 inhibitors". Molecular Diversity. 24: 1–14. doi:10.1007/s11030-019-10006-z.
- ↑ Hemasree, G. N. S., Satish, K. S., Rajalekshmi, S. G., Burri, R. R., & Murthy, T. P. K. (2024). "Exploration of interaction interface of TRKβ/BDNF through fingerprint analysis to disinter potential agonists". Molecular Diversity. 28: 1531–1549. doi:10.1007/s11030-023-10673-z.
- ↑ https://www.tiaft.org/past-meetings/tiaft2003/sci/O5_05.html
- ↑ Cody, J. T., & Valtier, S. (2001). "Amphetamine, clobenzorex, and 4-hydroxyclobenzorex levels following multidose administration of clobenzorex". Journal of Analytical Toxicology. 25 (3): 158–165. doi:10.1093/jat/25.3.158.
- ↑ Valtier, S., & Cody, J. T. (2000). "Differentiation of clobenzorex use from amphetamine abuse using the metabolite 4-hydroxyclobenzorex". Journal of Analytical Toxicology. 24 (7): 606–613. doi:10.1093/jat/24.7.606.
- ↑ Valtier, S., & Cody, J. T. (2000). "Differentiation of clobenzorex use from amphetamine abuse using the metabolite 4-hydroxyclobenzorex". Journal of Analytical Toxicology. 24 (7): 606–613. doi:10.1093/jat/24.7.606.
- ↑ Young, R., Darmani, N. A., Elder, E. L., Dumas, D., & Glennon, R. A. (1997). "Clobenzorex: Evidence for amphetamine-like behavioral actions". Pharmacology, Biochemistry, and Behavior. 56 (2): 311–316. doi:10.1016/S0091-3057(96)00329-2.
- ↑ Sinha, A., Lewis, O., Kumar, R., H. Yeruva, S. L., & Curry, B. H. (2016). "Adult ADHD Medications and Their Cardiovascular Implications". Case Reports in Cardiology. 2016: 2343691. doi:10.1155/2016/2343691. ISSN 2090-6404.
- ↑ Gabriela, C.-M., Adrian, H. H., Ángel, M.-G., Raúl, M.-Z., Rafael, G.-D., & Eleazar, L.-P. (2017). "Possible peripheral lipolytic effect of clobenzorex hydrochloride in patients with obesity". Medico Research Chronicles. 4 (3): 252–265. ISSN 2394-3971.
- ↑ J. Lozano-Cuenca, J., A. González-Hernández, A., López-Canales, O. A., Villagrana-Zesati, J. R., Rodríguez-Choreão, J. D., Morín-Zaragoza, R., Castillo-Henkel, E. F., & López-Canales, J. S. (2017). "Possible mechanisms involved in the vasorelaxant effect produced by clobenzorex in aortic segments of rats". Brazilian Journal of Medical and Biological Research. 50 (9). doi:10.1590/1414-431X20175765.
- ↑ Nutt DJ, King LA, Phillips LD (November 2010). "Drug harms in the UK: a multicriteria decision analysis". Lancet. 376 (9752): 1558–1565. CiteSeerX 10.1.1.690.1283
 . doi:10.1016/S0140-6736(10)61462-6. PMID 21036393. Unknown parameter
. doi:10.1016/S0140-6736(10)61462-6. PMID 21036393. Unknown parameter |s2cid=ignored (help) - ↑ Nutt, D., King, L. A., Saulsbury, W., Blakemore, C. (24 March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". The Lancet. 369 (9566): 1047–1053. doi:10.1016/S0140-6736(07)60464-4. ISSN 0140-6736.
- ↑ Human health effects - Amphetamine | http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@rn+@rel+300-62-9
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedNestler2009 - ↑ Carvalho, M., Carmo, H., Costa, V. M., Capela, J. P., Pontes, H., Remião, F., Carvalho, F., Bastos, M. de L. (1 August 2012). "Toxicity of amphetamines: an update". Archives of Toxicology. 86 (8): 1167–1231. doi:10.1007/s00204-012-0815-5. ISSN 1432-0738.
- ↑ Miyazaki, I., Asanuma, M. (June 2008). "Dopaminergic neuron-specific oxidative stress caused by dopamine itself". Acta Medica Okayama. 62 (3): 141–150. doi:10.18926/AMO/30942. ISSN 0386-300X.
- ↑ Advokat, C. (July 2007). "Literature Review: Update on Amphetamine Neurotoxicity and Its Relevance to the Treatment of ADHD". Journal of Attention Disorders. 11 (1): 8–16. doi:10.1177/1087054706295605. ISSN 1087-0547.
- ↑ Bowyer, J. F., Hanig, J. P. (14 November 2014). "Amphetamine- and methamphetamine-induced hyperthermia: Implications of the effects produced in brain vasculature and peripheral organs to forebrain neurotoxicity". Temperature: Multidisciplinary Biomedical Journal. 1 (3): 172–182. doi:10.4161/23328940.2014.982049. ISSN 2332-8940.
- ↑ Chetsawang, J., Mukda, S., Srimokra, R., Govitrapong, P., Chetsawang, B. (3 July 2017). "Role of Melatonin in Reducing Amphetamine-Induced Degeneration in Substantia Nigra of Rats via Calpain and Calpastatin Interaction". Journal of Experimental Neuroscience. 11: 1179069517719237. doi:10.1177/1179069517719237. ISSN 1179-0695.
- ↑ Leeboonngam, T., Pramong, R., Sae-Ung, K., Govitrapong, P., Phansuwan-Pujito, P. (April 2018). "Neuroprotective effects of melatonin on amphetamine-induced dopaminergic fiber degeneration in the hippocampus of postnatal rats". Journal of Pineal Research. 64 (3). doi:10.1111/jpi.12456. ISSN 1600-079X.
- ↑ Young, R., Darmani, N. A., Elder, E. L., Dumas, D., & Glennon, R. A. (1997). "Clobenzorex: Evidence for amphetamine-like behavioral actions". Pharmacology, Biochemistry, and Behavior. 56 (2): 311–316. doi:10.1016/S0091-3057(96)00329-2.
- ↑ 36.0 36.1 36.2 Shoptaw, S. J., Kao, U., Ling, W. (21 January 2009). Cochrane Drugs and Alcohol Group, ed. "Treatment for amphetamine psychosis". Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD003026.pub3. ISSN 1465-1858.
- ↑ Hofmann, F. G. (1983). A handbook on drug and alcohol abuse: the biomedical aspects (2nd ed ed.). Oxford University Press. ISBN 9780195030563.
- ↑ http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf
- ↑ Greenwald, M. K., Lundahl, L. H., Steinmiller, C. L. (December 2010). "Sustained Release d-Amphetamine Reduces Cocaine but not 'Speedball'-Seeking in Buprenorphine-Maintained Volunteers: A Test of Dual-Agonist Pharmacotherapy for Cocaine/Heroin Polydrug Abusers". Neuropsychopharmacology. 35 (13): 2624–2637. doi:10.1038/npp.2010.175. ISSN 0893-133X.
- ↑ Siciliano, C. A., Saha, K., Calipari, E. S., Fordahl, S. C., Chen, R., Khoshbouei, H., Jones, S. R. (10 January 2018). "Amphetamine Reverses Escalated Cocaine Intake via Restoration of Dopamine Transporter Conformation". The Journal of Neuroscience. 38 (2): 484–497. doi:10.1523/JNEUROSCI.2604-17.2017. ISSN 0270-6474.
- ↑ Krystal, J. H., Perry, E. B., Gueorguieva, R., Belger, A., Madonick, S. H., Abi-Dargham, A., Cooper, T. B., MacDougall, L., Abi-Saab, W., D’Souza, D. C. (1 September 2005). "Comparative and Interactive Human Psychopharmacologic Effects of Ketamine and Amphetamine: Implications for Glutamatergic and Dopaminergic Model Psychoses and Cognitive Function". Archives of General Psychiatry. 62 (9): 985. doi:10.1001/archpsyc.62.9.985. ISSN 0003-990X.
- ↑ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27. Unknown parameter
|url-status=ignored (help) - ↑ "Controlled Drugs and Substances Act (S.C. 1996, c. 19): SCHEDULE I". Department of Justice Canada. Retrieved 2023-05.
- ↑ "Misuse of Drugs Act 1971 (c. 38): SCHEDULE 2: Controlled Drugs". Office of Public Sector Information. Retrieved 2009-06-15.
- ↑ Boos, Terrence (April 6, 2023). "Clobenzorex Letter". Imgur. Archived from the original on July 11, 2023. Retrieved July 11, 2023.
- ↑ "Is it legal for me to personally import drugs?". FDA.gov. Food and Drug Administration. 28 June 2021. Retrieved 22 July 2021.
- ↑ "World Anti-Doping Code International Standard Prohibited List 2023" (PDF). World Anti-Doping Agency. September 2022.
