GHB

Fatal overdose may occur when GABAergic substances are combined with other depressants such as opiates, benzodiazepines, barbiturates, gabapentinoids, thienodiazepines or alcohol.[1]
It is strongly discouraged to combine these substances, particularly in common to heavy doses.
| Summary sheet: GHB |
| GHB | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||
| Common names | GHB, G, Xyrem, Sodium oxybate | ||||||||||||||||||||||||||||||||
| Substitutive name | gamma-Hydroxybutyric acid | ||||||||||||||||||||||||||||||||
| Systematic name | 4-Hydroxybutanoic acid | ||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||
| Psychoactive class | Depressant | ||||||||||||||||||||||||||||||||
| Chemical class | γ-Hydroxy acid | ||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||
| Nitrous | |||||||||||||||||||||||||||||||||
| Amphetamines | |||||||||||||||||||||||||||||||||
| MDMA | |||||||||||||||||||||||||||||||||
| Cocaine | |||||||||||||||||||||||||||||||||
| Ketamine | |||||||||||||||||||||||||||||||||
| MXE | |||||||||||||||||||||||||||||||||
| DXM | |||||||||||||||||||||||||||||||||
| PCP | |||||||||||||||||||||||||||||||||
| Alcohol | |||||||||||||||||||||||||||||||||
| Opioids | |||||||||||||||||||||||||||||||||
| Tramadol | |||||||||||||||||||||||||||||||||
| Benzodiazepines | |||||||||||||||||||||||||||||||||
gamma-Hydroxybutyric acid (also known as 4-hydroxybutanoic acid and GHB) is a depressant substance. It is found naturally as a neurotransmitter and is also a precursor to GABA, glutamate, and glycine in certain brain areas. It acts on the GHB receptor and is a weak agonist at the GABAB receptor.
GHB as the sodium salt, known by the trade name Xyrem,[3] is a prescription sleep-aid which is used to treat various medical conditions such as cataplexy[4] and excessive daytime sleepiness in patients with narcolepsy.[5] It has also been used in a medical setting as a general anesthetic to treat conditions such as insomnia, clinical depression, and alcoholism,[6] and to improve athletic performance.
GHB is used as a recreational substance for its alcohol-like effects. While a common recreational dose is between 1.5 - 2.5 grams, a dose between 2.5 grams and 5 grams will likely result in falling asleep within 5 - 15 minutes, and a dose of 5 - 10 grams can result in convulsions, unconsciousness (a coma-like state) and vomiting. Doses above 10 grams are associated with a risk of death.[2] Do not confuse grams with milliliters (the density of GHB is aproximately 1.2g/ml [7]). If consuming GHB that has already been premixed into a liquid form, additional caution is required as there is no way to know for sure the concentration of the solution (e.g. how many grams of GHB is in each mL of solution). As such, users are advised to start with a low dose and work their way up slowly by increasing the dose in small increments. However, an appropriate interval between dosing is essential to avoid accidental overdose.
GHB, along with GBL, has acquired a reputation as a "date rape drug," in which it is purportedly secretly placed into alcoholic drinks.[citation needed] There is very limited evidence to suggest that this actually happens but care should always be taken when accepting drinks from strangers.
Chemistry
GHB, or gamma-Hydroxybutanoic acid, is a carboxylic acid substituted with an additional hydroxy group. GHB contains a four carbon chain with a terminal carbon bonded to a hydroxy group (OH-) and double bonded to an oxygen group to form a carboxyl unit; this is butanoic acid. At the other end of the four carbon change at Rγ, GHB is substituted with a hydroxy group.
GHB is found naturally in the human central nervous system as well as in wine, beef, some citrus fruits, and in almost all animals (in small amounts).[8]
Pharmacology
GHB has at least two distinct binding sites[9] in the central nervous system. GHB is an agonist at the newly characterized GHB receptor, which is excitatory.[10][11] It is also a weak agonist at the GABAB receptor, which is inhibitory.[11]
However, at therapeutic doses, GHB reaches much higher concentrations in the brain and activates GABAB receptors, which are primarily responsible for its sedative effects.[12] GHB's sedative effects are blocked by GABAB antagonists. As the GABA system is the most prolific inhibitory receptor set within the brain, its modulation results in the sedating (or calming effects) of GHB on the nervous system. While research into the GHB receptor is limited, there is evidence that activation of the GHB receptor in some brain areas results in the release of glutamate, the principal excitatory neurotransmitter.[13] Drugs that selectively activate the GHB receptor cause absence seizures in high doses, as do GHB and GABAB agonists.[13]
Activation of both the GHB receptor and GABAB is responsible for the addictive profile of GHB. GHB's effect on dopamine release is biphasic.[12] This means that while low concentrations stimulate dopamine release via the GHB receptor,[14] higher concentrations inhibit dopamine release via GABAB receptors.[15] After an initial phase of inhibition, dopamine release is then increased via the GHB receptor.
GHB induces the accumulation of either a derivative of tryptophan or tryptophan itself, possibly by increasing tryptophan transport across the blood–brain barrier. GHB-induced stimulation may be due to this increase in tryptophan transport to the brain and in its uptake by serotonergic cells. As the serotonergic system may be involved in the regulation of sleep, mood, and anxiety, the stimulation of this system by high doses of GHB may be involved in certain neuropharmacological events induced by GHB administration.
These findings may explain the paradoxical mix of sedative and stimulatory properties of GHB as well as the so-called "rebound" effect reported by individuals using GHB as a sleeping agent wherein they awake suddenly after several hours of GHB-induced deep sleep. Over time, the concentration of GHB in the system decreases below the threshold for significant GABAB receptor activation and activates predominantly the GHB receptor, leading to wakefulness.
Subjective effects
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Stimulation & Sedation - At lower doses, GHB is physically stimulating, encouraging movement and wakefulness. At higher doses, however, it becomes physically sedating, encouraging sleep and lethargy.
- Respiratory depression - In cases of GHB overdoses, many reportedly experience an abnormal pattern of breathing characterized by progressively deeper and sometimes faster breathing followed by a gradual decrease that results in a temporary stop in breathing called an apnea.
- Muscle relaxation - GHB induces profound muscle relaxation similar to, but more intense than, the relaxation produced by benzodiazepines and alcohol. This muscle relaxation is accompanied with a general loss of motor control, which may put the user at increased risk of physical injury.
- Dehydration
- Dizziness - Dizziness is a common side effect of GHB and can occur with normal recreational doses. Users may also suddenly feel light-headed or faint, and feel a strong urge to lie down. Colloquially known as "the spins".
- Motor control loss
- Nausea
- Increased salivation - Increased salivation is very common.
- Stomach cramps
- Muscle cramps
- Optical sliding
- Pupil dilation
- Vasodilation
- Headaches
- Seizure - Very high dosages of GHB have consistently produced convulsions.
- Orgasm suppression - Can occur at high doses or with multiple redosing.
Cognitive effects 
-
- Sleepiness & Wakefulness - At very low dosages, GHB can make one tired, while common doses are primarly wakefullness-promoting. High doses can lead to feelings of being extremely sleepy.
- Analysis suppression
- Anxiety suppression
- Cognitive euphoria - GHB produces intense states of euphoria comparable to that of cocaine, MDMA, and opiates. For this reason, it is sometimes called "liquid ecstasy". It is commonly described as a more euphoric and disinhibiting version of alcohol. While this may be accurate, it is also far easier to overdose on.
- Compulsive redosing - This is somewhat prevalent due to its short duration and rebound anxiety.
- Creativity enhancement - Creativity enhancement is most apparent at low to common doses.
- Disinhibition - Social and behavioral disinhibition on GHB is extremely powerful and perhaps its most prominent feature. Users are more liable to exhibit impaired decision-making and engage in risky activities such as unprotected sex or unsafe substance combinations. Users are advised to only take GHB in safe environments with people they trust not to take advantage of their vulnerable state.
- Dream potentiation - GHB can have a very pronounced effect on dream recall and vividness. Conversely, discontinuation can result in bad dreams or nightmares. Additionally, intoxication on GBL can lead to one seeing their own dream being manifested, similar to a wake-induced lucid dream (WILD) and simultaneous states of wakefulness and dreaming.
- Empathy, affection, and sociability enhancement - GHB presents strong entactogenic effects which, although weaker than that of MDMA, are still prominent and well defined.
- Immersion enhancement
- Increased libido - GBL reportedly produces less increases of libido than GHB does.
- Increased music appreciation
- Introspection
- Rejuvenation
- Suggestibility enhancement
- Memory suppression
- Motivation enhancement & Motivation suppression - As with other coinciding effects, common doses can lead to greater motivation whilst high doses generally result in lethargy.
- Spatial disorientation - Disorientation is very common in dark areas.
- Suggestibility enhancement
- Thought acceleration or Thought deceleration - Low to common doses are primarly stimulating and one finds themselves constantly generating thoughts.
Auditory effects 
After effects 
-
- Rebound anxiety - Rebound anxiety is a commonly observed effect with anxiolytic substances like GHB. This, along with GHB's short duration of action can lead to compulsive redosing.
Experience reports
There are currently no anecdotal reports which describe the effects of this compound within our experience index. Additional experience reports can be found here:
Toxicity and harm potential

GHB is considered to be a safe and non-toxic substance when used responsibly or medically. The LD50 is above the active dosage, and there is no danger of acute toxicity. However, it can become dangerous when used as a recreational drug or abused. There have been many negative reports from recreational users who have overdosed, combined GHB with alcohol or other drugs, or accidentally dosed themselves unexpectedly.[18]
One publication investigated 226 deaths attributed to GHB.[19] Seventy-one deaths (34%) were caused by GHB alone while the other deaths were from respiratory depression caused by interaction with alcohol or other drugs.
As an endogenous regulator of energy metabolism and a natural neurotransmitter, GHB is well-known to the brain and organs which are used to its effects and have highly efficient systems for metabolizing it safely. The substance is eliminated (that is, back to baseline levels) in 2-4 hours and continues to be so even after twice-daily doses for a week.[20] In one European study, no adverse effects were reported after several years of regular recreational use.[21]
Accidental ingestions of GHB have also occurred due to inadequate storage methods. If GHB is put into a clear liquid, glass, or bottle, it can be easily mistaken for water. It is recommended to clearly label your GHB in writing and dye the liquid with blue food coloring so it no longer resembles a drinkable beverage. It is also recommended to store your GHB in a container that no one would drink out of.
GHB is also corrosive it is recommend it to dilute it with a non-alcoholic liquid in the ratio of atleast 1:100ml if not done this can result in burns in the intestine as well as the mouth.[1]
It is strongly recommended that one use harm reduction practices when using this substance.
Neurotoxicity
A 2022 review[22] compared 43 studies on GHB-induced cognitive impairment in humans and animals. The analysis suggests that moderate or clinical use may result in acute cognitive impairment. Working memory, short-term memory, and impaired performance on cognitive tasks were impaired after doses as low as 10mg/kg, but these effects appear to be temporary. Conversely, one study found that a dose of 20mg/kg improved social and non-social cognition, but did not affect base cognitive functions, such as visual or verbal memory recall. However, chronic heavy use of GHB, especially if accompanied by GHB-induced comas appear to cause long-term cognitive impairment and are likely neurotoxic.
In multiple studies, GHB has been found to impair spatial memory, working memory, learning and memory in rats with chronic administration.[23][24][25][26] These effects are associated with decreased NMDA receptor expression in the cerebral cortex and possibly other areas as well.[27]
One study found that repeated administration of GHB to rats for 15 days drastically reduced the number of neurons and non-neuronal cells within the hippocampus and in the prefrontal cortex. With doses of 10 mg/kg of GHB, they were decreased by 61% in the hippocampus region and 32% in the prefrontal cortex, and with 100 mg/kg, they were decreased by 38% and 9%, respectively. This paper demonstrates contradicting effects on neuronal loss, with lower doses (10 mg/kg) producing the most neurotoxicity, and higher doses (100 mg/kg) producing less.
Overdose
To avoid a possible overdose of GHB, it is important to start with a low dose and work your way up slowly by increasing the dosage in small increments. While a common recreational dose is 3g, a dose of 5g - 10g can result in convulsions, unconsciousness and vomiting. Doses above 10 grams are associated with a risk of death.[2] One must also factor in the added difficulty of knowing the purity of the product (among other problems like its hygroscopy may lower the concentration of GHB in one solution, which is the form which is commonly bought and sold in the illicit market). This makes it hard for even the experienced user to dose properly.[28]
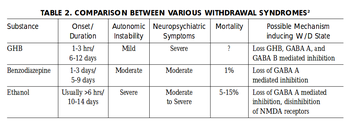
Tolerance and addiction potential
GHB is moderately to highly physically and moderately psychologically addictive. The frequent use of GHB can cause withdrawal symptoms similar to those caused by other depressants such as alcohol and benzodiazepines if abruptly discontinued.[30][31] These symptoms seem to depend on the dosage and the length of time the drug was used for. Light to moderate users often experience anxiety, insomnia, sleep-related problems, and tremors whereas heavy use can cause severe withdrawal symptoms like delirium, psychosis, and hallucinations.[32][29]
GHB, when used medicinally (two medicinal doses per day, four hours apart), creates little to no dependance while the more frequent one uses GHB, it causes exponentially more dependance and withdrawal symptoms.[33]
Although there have been reported fatalities due to GHB withdrawal, reports are inconclusive and further research is needed.[34]
Tolerance will develop to the sedative-hypnotic effects within several days of continuous use. After cessation, the tolerance returns to baseline in 7 - 14 days. Withdrawal symptoms or rebound symptoms may occur after ceasing usage abruptly following a few weeks or longer of steady dosing, and may necessitate a gradual dose reduction to minimize neurotoxicity from withdrawal. It is proposed that GHB and especially GBL can lead to dependence at a significantly faster rate than longer-acting depressants.
GHB presents cross-tolerance with 1,4-Butanediol and GBL, since these act as prodrugs for GHB. Cross-tolerance with alcohol and baclofen has been shown in rats,[35][36] and is likely with other GABAB-agonists.
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- Nitrous - Both substances potentiate the ataxia and sedation caused by the other and can lead to unexpected loss of consciousness at high doses. While unconscious, vomit aspiration is a risk if not placed in the recovery position. Memory blackouts are likely.
- Amphetamines - Stimulants increase respiration rate allowing a higher dose of sedatives. If the stimulant wears off first then the GHB may overcome the patient and cause respiratory arrest.
- MDMA - Large amounts of GHB/GBL may overwhelm the effects of MDMA on the comedown.
- Cocaine - Stimulants increase respiration rate allowing a higher dose of sedatives. If the stimulant wears off first then the GHB may overcome the patient and cause respiratory arrest. Likewise the GHB can wear off and leave a dangerous concentration of Cocaine behind.
- Ketamine - Both substances cause ataxia and bring a risk of vomiting and unconsciousness. If the user falls unconscious while under the influence there is a severe risk of vomit aspiration if they are not placed in the recovery position.
- MXE - Both substances cause ataxia and bring a risk of vomiting and unconsciousness. If the patient falls unconscious while under the influence there is a severe risk of vomit aspiration if they are not placed in the recovery position.
- DXM - Both substances cause ataxia and bring a risk of vomiting and unconsciousness. If the patient falls unconscious while under the influence there is a severe risk of vomit aspiration if they are not placed in the recovery position. This combination is hard to predict.
- PCP - Details of this combination are not well understood but PCP generally interacts in an unpredictable manner.
- Alcohol - Even in very low doses this combination rapidly leads to memory loss, severe ataxia and unconsciousness. There is a high risk of vomit aspiration while unconscious.
- Opioids - The two substances potentiate each other strongly and unpredictably, very rapidly leading to unconsciousness. While unconscious, vomit aspiration is a risk if not placed in the recovery position.
- Tramadol - The sedative effects of this combination can lead to dangerous respiratory depression.
- Benzodiazepines - The two substances potentiate each other strongly and unpredictably, very rapidly leading to unconsciousness. While unconscious, vomit aspiration is a risk if not placed in the recovery position.
Legal status
In 2001, GHB was placed under international control, in Schedule IV of the 1971 UN Convention on Psychotropic Substances.[37]
- Austria: GHB is illegal to possess, produce and sell under the SMG (Suchtmittelgesetz Österreich).[citation needed]
- Australia: GHB, 1,4-B and GBL are all Class B illegal drugs, along with any possible esters, ethers and aldehydes.[citation needed]
- Chile: GHB is a controlled drug under the law "Ley de substancias psicotropicas y estupefacientes" (psychotropic substances and narcotics).[citation needed]
- Canada: GHB is controlled under Schedule I of the Controlled Drugs and Substances Act. Activities such as sale, possession and production of GHB are illegal, unless authorized for medical, scientific or industrial purposes.[38]
- Germany: GHB is a controlled substance under Anlage III of the BtMG. It can only be prescribed on a narcotic prescription form. There is an exception for injection preperations, containing up to 20% or 2g GHB per unit, which are viewed as a regular medicine.[39]
- Italy: GHB is a schedule IV drug (Tabella 4) of the "Testo unico sulla droga (D.P.R. 309/90)". When prescribed for medical use it falls under Pharmaceuticals section B (Tabella medicinali sezione B).[40][41]
- Hong Kong: GHB is regulated under Schedule 1 of Hong Kong's Chapter 134 Dangerous Drugs Ordinance.[citation needed]
- New Zealand: GHB, 1,4-B and GBL are all Class B illegal drugs, along with any possible esters, ethers and aldehydes.[citation needed]
- Norway: GHB is considered a narcotic and is only available by prescription under the trade name Xyrem.[citation needed]
- Romania: GHB is considered a Schedule I illegal drug.[42]
- Switzerland: GHB is a controlled substance specifically named under Verzeichnis A. Medicinal use is permitted.[43]
- United Kingdom: GHB was made a Class C drug in June 2003.[citation needed]
- United States: GHB was placed on Schedule I of the Controlled Substances Act in March 2000. However, when sold as sodium oxybate, it is considered a Schedule III substance but with Schedule I trafficking penalties.[44] It is one of several drugs that are listed in multiple schedules.
See also
External links
References
- ↑ Risks of Combining Depressants - TripSit
- ↑ 2.0 2.1 2.2 Erowid GHB Vault : Dosage
- ↑ http://www.reuters.com/finance/stocks/companyProfile?rpc=66&symbol=JAZZ.O
- ↑ Sodium Oxybate: MedlinePlus Drug Information
- ↑ Kluger, R., Mamelak, M., Pharmaceutical composition and treatment of narcolepsy
- ↑ Gessa, G. L., Fadda, F., Campochiaro, C. M. di, Use of gamma-hydroxybutyric acid salts for preparing pharmaceutical compositions for use in the treatment of alcoholism, and the compositions obtained
- ↑ https://www.chemspider.com/Chemical-Structure.9984.html
- ↑ Weil, A., Rosen, W., Weil, A. (1993). From chocolate to morphine: everything you need to know about mind-altering drugs (Rev. and updated ed.). Houghton Mifflin. ISBN 9780395660799.
- ↑ Mamelak, M. (1 December 1989). "Gammahydroxybutyrate: An endogenous regulator of energy metabolism". Neuroscience & Biobehavioral Reviews. 13 (4): 187–198. doi:10.1016/S0149-7634(89)80053-3. ISSN 0149-7634.
- ↑ Wu, Y., Ali, S., Ahmadian, G., Liu, C. C., Wang, Y. T., Gibson, K. M., Calver, A. R., Francis, J., Pangalos, M. N., Carter Snead, O. (1 December 2004). "γ-Hydroxybutyric acid (GHB) and γ-aminobutyric acidB receptor (GABABR) binding sites are distinctive from one another: molecular evidence". Neuropharmacology. 47 (8): 1146–1156. doi:10.1016/j.neuropharm.2004.08.019. ISSN 0028-3908.
- ↑ 11.0 11.1 Maitre, M., Humbert, J.-P., Kemmel, V., Aunis, D., Andriamampandry, C. (1 March 2005). "Mécanismes d'action d'un médicament détourné : le γ-hydroxybutyrate". médecine/sciences. 21 (3): 284–289. doi:10.1051/medsci/2005213284. ISSN 0767-0974.
- ↑ 12.0 12.1 Dimitrijevic, N., Dzitoyeva, S., Satta, R., Imbesi, M., Yildiz, S., Manev, H. (20 September 2005). "Drosophila GABAB receptors are involved in behavioral effects of γ-hydroxybutyric acid (GHB)". European Journal of Pharmacology. 519 (3): 246–252. doi:10.1016/j.ejphar.2005.07.016. ISSN 0014-2999.
- ↑ 13.0 13.1 Castelli, M. P., Ferraro, L., Mocci, I., Carta, F., Carai, M. A. M., Antonelli, T., Tanganelli, S., Cignarella, G., Gessa, G. L. (19 September 2003). "Selective γ-hydroxybutyric acid receptor ligands increase extracellular glutamate in the hippocampus, but fail to activate G protein and to produce the sedative/hypnotic effect of γ-hydroxybutyric acid: γ-Hydroxybutyric analogues on glutamate levels". Journal of Neurochemistry. 87 (3): 722–732. doi:10.1046/j.1471-4159.2003.02037.x. ISSN 0022-3042.
- ↑ Maitre, M., Hechler, V., Vayer, P., Gobaille, S., Cash, C. D., Schmitt, M., Bourguignon, J. J. (November 1990). "A specific gamma-hydroxybutyrate receptor ligand possesses both antagonistic and anticonvulsant properties". The Journal of Pharmacology and Experimental Therapeutics. 255 (2): 657–663. ISSN 0022-3565.
- ↑ Smolders, I., De Klippel, N., Sarre, S., Ebinger, G., Michotte, Y. (15 September 1995). "Tonic GABA-ergic modulation of striatal dopamine release studied by in vivo microdialysis in the freely moving rat". European Journal of Pharmacology. 284 (1): 83–91. doi:10.1016/0014-2999(95)00369-V. ISSN 0014-2999.
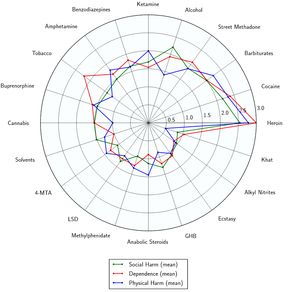
- ↑ Nutt DJ, King LA, Phillips LD (November 2010). "Drug harms in the UK: a multicriteria decision analysis". Lancet. 376 (9752): 1558–1565. CiteSeerX 10.1.1.690.1283
 . doi:10.1016/S0140-6736(10)61462-6. PMID 21036393. Unknown parameter
. doi:10.1016/S0140-6736(10)61462-6. PMID 21036393. Unknown parameter |s2cid=ignored (help) - ↑ Nutt, D., King, L. A., Saulsbury, W., Blakemore, C. (24 March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". The Lancet. 369 (9566): 1047–1053. doi:10.1016/S0140-6736(07)60464-4. ISSN 0140-6736.
- ↑ GHB - Erowid Exp - “GHB Overdoses & Poisonings”
- ↑ Zvosec, D. L., Smith, S. W., Porrata, T., Strobl, A. Q., Dyer, J. E. (March 2011). "Case series of 226 γ-hydroxybutyrate-associated deaths: lethal toxicity and trauma". The American Journal of Emergency Medicine. 29 (3): 319–332. doi:10.1016/j.ajem.2009.11.008. ISSN 1532-8171.
- ↑ Ferrara, S., Zotti, S., Tedeschi, L., Frison, G., Castagna, F., Gallimberti, L., Gessa, G., Palatini, P. (September 1992). "Pharmacokinetics of gamma-hydroxybutyric acid in alcohol dependent patients after single and repeated oral doses". British Journal of Clinical Pharmacology. 34 (3): 231–235. doi:10.1111/j.1365-2125.1992.tb04129.x. ISSN 0306-5251.
- ↑ Laborit, H. (January 1972). "Correlations between protein and serotonin synthesis during various activities of the central nervous system (slow and desynchronized sleep, learning and memory, sexual activity, morphine tolerance, aggressiveness, and pharmacological action of sodium gamma-hydroxybutyrate)". Research Communications in Chemical Pathology and Pharmacology. 3 (1): 51–81. ISSN 0034-5164.
- ↑ Amsterdam JV, Brunt TM, Pereira FR, Crunelle CL, Brink WVD. Cognitive Impairment Following Clinical or Recreational Use of Gammahydroxybutyric Acid (GHB): A Systematic Review. Curr Neuropharmacol. 2022;20(4):809-819.
- ↑ Sircar, R., Basak, A. (1 December 2004). "Adolescent γ-hydroxybutyric acid exposure decreases cortical N-methyl-d-aspartate receptor and impairs spatial learning". Pharmacology Biochemistry and Behavior. 79 (4): 701–708. doi:10.1016/j.pbb.2004.09.022. ISSN 0091-3057.
- ↑ García, F. B., Pedraza, C., Arias, J. L., Navarro, J. F. (August 2006). "[Effects of subchronic administration of gammahydroxybutyrate (GHB) on spatial working memory in rats]". Psicothema. 18 (3): 519–524. ISSN 0214-9915.
- ↑ Sircar, R., Basak, A., Sircar, D. (October 2008). "γ-Hydroxybutyric Acid-Induced Cognitive Deficits in the Female Adolescent Rat". Annals of the New York Academy of Sciences. 1139 (1): 386–389. doi:10.1196/annals.1432.044. ISSN 0077-8923.
- ↑ Pedraza, C., García, F. B., Navarro, J. F. (October 2009). "Neurotoxic effects induced by gammahydroxybutyric acid (GHB) in male rats". The International Journal of Neuropsychopharmacology. 12 (09): 1165. doi:10.1017/S1461145709000157. ISSN 1461-1457.
- ↑ Sircar, R., Basak, A. (December 2004). "Adolescent gamma-hydroxybutyric acid exposure decreases cortical N-methyl-D-aspartate receptor and impairs spatial learning". Pharmacology, Biochemistry, and Behavior. 79 (4): 701–708. doi:10.1016/j.pbb.2004.09.022. ISSN 0091-3057.
- ↑ https://www.erowid.org/chemicals/ghb/ghb_health.shtml
- ↑ 29.0 29.1 GHB Withdrawal Syndrome | Texas Commission on Alcohol and Drug Abuse | https://www.erowid.org/chemicals/ghb/ghb_addiction2.pdf
- ↑ Kim, S. Y., Barker, J. C., Anderson, I. B., Dyer, J. E., Earnest, G., Blanc, P. D. (2008). "Systematic Assessment of Gamma Hydroxybutyrate (GHB) Effects During and After Acute Intoxication". The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 17 (4): 312–318. doi:10.1080/10550490802138988. ISSN 1055-0496.
- ↑ Carter, L. P., Pardi, D., Gorsline, J., Griffiths, R. R. (1 September 2009). "Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem®): differences in characteristics and misuse". Drug and alcohol dependence. 104 (1–2): 1–10. doi:10.1016/j.drugalcdep.2009.04.012. ISSN 0376-8716.
- ↑ Dyer, J. E., Roth, B., Hyma, B. A. (February 2001). "Gamma-hydroxybutyrate withdrawal syndrome". Annals of Emergency Medicine. 37 (2): 147–153. doi:10.1067/mem.2001.112985. ISSN 0196-0644.
- ↑ https://drugs-forum.com/threads/important-ghb-gbl-addiction-withdrawal.43390/page-4
- ↑ Galloway, G. P., Frederick, S. L., Staggers, F. E., Gonzales, M., Stalcup, S. A., Smith, D. E. (January 1997). "Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence". Addiction. 92 (1): 89–96. doi:10.1111/j.1360-0443.1997.tb03640.x. ISSN 0965-2140.
- ↑ Colombo, G., Agabio, R., Lobina, C., Reali, R., Fadda, F., Gessa, G. L. (6 February 1995). "Cross-tolerance to ethanol and γ-hydroxybutyric acid". European Journal of Pharmacology. 273 (3): 235–238. doi:10.1016/0014-2999(94)00687-3. ISSN 0014-2999.
- ↑ Smith, M. A., Gergans, S. R., Lyle, M. A. (15 December 2006). "The motor-impairing effects of GABAA and GABAB agonists in γ-hydroxybutyrate (GHB)-treated rats: Cross-tolerance to baclofen but not flunitrazepam". European Journal of Pharmacology. 552 (1): 83–89. doi:10.1016/j.ejphar.2006.08.080. ISSN 0014-2999.
- ↑ "Control measures". European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Retrieved October 20, 2020.
- ↑ Canada, H. (2012), GHB
- ↑ Anlage III BtMG - Einzelnorm
- ↑ Tabella IV Sostanze stupefacenti http://www.salute.gov.it/imgs/C_17_pagineAree_3729_listaFile_itemName_3_file.pdf
- ↑ Tabella Medicinali D.P.R. 309/90 http://www.salute.gov.it/imgs/C_17_pagineAree_3729_listaFile_itemName_4_file.xls
- ↑ https://legislatie.just.ro/Public/DetaliiDocument/23629
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
- ↑ Laws, 2010