Temazepam

Fatal overdose may occur when benzodiazepines are combined with other depressants such as opiates, barbiturates, gabapentinoids, thienodiazepines, alcohol or other GABAergic substances.[1]
It is strongly discouraged to combine these substances, particularly in common to heavy doses.
| Summary sheet: Temazepam |
| Temazepam | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||
| Common names | Temazepam, Restoril, Normison | ||||||||||||||||||||||||||||
| Substitutive name | Temazepam | ||||||||||||||||||||||||||||
| Systematic name | 7-Chloro-1,3-dihydro-3-hydroxy-1-methyl-5-phenyl-1,4-benzodiazepin-2-one | ||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||
| Psychoactive class | Depressant | ||||||||||||||||||||||||||||
| Chemical class | Benzodiazepine | ||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||
Temazepam (trade name Restoril) is a short-acting[3] psychoactive substance of the benzodiazepine class which produces anxiolytic, sedative, hypnotic, muscle relaxant, anticonvulsant, and amnesic effects.[4] Temazepam, like other benzodiazepines, binds to specific sites on the GABAA gamma-amino-butyric acid receptor.
Temazepam is used for the short term treatment of severe and debilitating insomnia and as a preoperative sedative. Oral administration of temazepam in humans resulted in rapid absorption, a fast onset of action and symptomatic relief. Significant blood levels were achieved in 15-30 minutes post-ingestion with peak levels achieved within 30 minutes to 3 three hours.[5] Temazepam is effective for both inducing sleep and for maintaining sleep.
It's worth noting that the sudden discontinuation of benzodiazepines can be potentially dangerous or life-threatening for individuals using regularly for extended periods of time, sometimes resulting in seizures or death.[6] It is highly recommended to taper one's dose by gradually lowering the amount taken each day for a prolonged period of time instead of stopping abruptly.[7]
Chemistry
Temazepam is a drug of the benzodiazepine class. Benzodiazepine drugs contain a benzene ring fused to a diazepine ring, which is a seven membered ring with the two nitrogen constituents located at R1 and R4. The benzyl ring of temazepam is substituted at R8 with a chlorine group. Further, the diazepine ring is bonded at R5 to a phenyl ring. Temazepam also has a hydroxyl group bonded to the 3-position of the diazepine ring.
Temazepam is a white, crystalline substance, very slightly soluble in water, and sparingly soluble in alcohol.
Pharmacology
Benzodiazepines produce a variety of effects by binding to the benzodiazepine receptor site and magnifying the efficiency and effects of the neurotransmitter gamma aminobutyric acid (GABA) by acting on its receptors.[8] As this site is the most prolific inhibitory receptor set within the brain, its modulation results in the sedating (or calming effects) of temazepam on the nervous system. When temazepam binds to the GABAA receptor, it causes the Cl- ion pore to open more frequently. The anticonvulsant properties of benzodiazepines may be, in part or entirely, due to binding to voltage-dependent sodium channels rather than benzodiazepine receptors.[9]
Its main pharmacological action is to increase the effect of the neurotransmitter gamma-aminobutyric acid (GABA) at the GABAA receptor. This causes sedation, motor impairment, ataxia, anxiolysis, an anticonvulsant effect, muscle relaxation, and a reinforcing effect. As a medication before surgery, temazepam decreased cortisol in elderly patients. In rats, it triggered the release of vasopressin into paraventricular nucleus of the hypothalamus and decreased the release of ACTH under stress.[10]
In a single- and multiple-dose absorption, distribution, metabolism, and excretion (ADME) study, using tritium-labelled drug, temazepam was well absorbed and found to have minimal (8%) first-pass drug metabolism. No active metabolites were formed and the only significant metabolite present in blood was the O-conjugate. The unchanged drug was 96% bound to plasma proteins. The blood-level decline of the parent drug was biphasic, with the short half-life ranging from 0.4-0.6 hours and the terminal half-life from 3.5–18.4 hours (mean 8.8 hours), depending on the study population and method of determination.[3]
Subjective effects
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Sedation - In terms of energy level alterations, this drug has the potential to be extremely sedating and often results in an overwhelmingly lethargic state. At higher levels, this causes users to suddenly feel as if they are extremely sleep deprived and have not slept for days, forcing them to sit down and generally feel as if they are constantly on the verge of passing out instead of engaging in physical activities. This sense of sleep deprivation increases proportional to dosage and eventually becomes powerful enough to force a person into complete unconsciousness.
- Dizziness
- Muscle relaxation
- Motor control loss
- Respiratory depression
- Seizure suppression
- Decreased heart rate
- Perception of bodily heaviness - Temazepam is known to cause feelings of heaviness in the body. This effect can range from motor impairment and difficulty moving at lower doses to complete lethargy or inability to stand up or move at high doses.
- Physical euphoria - The euphoria felt on temazepam is significantly stronger than that felt on other benzodiazepines such as alprazolam.
Paradoxical effects 
- Paradoxical reactions to benzodiazepines such as increased seizures (in epileptics), aggression, increased anxiety, violent behavior, loss of impulse control, irritability and suicidal behavior sometimes occur (although they are rare in the general population, with an incidence rate below 1%).[11][12] These paradoxical effects occur with greater frequency in recreational abusers, individuals with mental disorders, children, and patients on high-dosage regimes.[13][14]
Cognitive effects 
-
The cognitive effects of temazepam can be broken down into several components which progressively intensify proportional to dosage. The general head space of temazepam is described by many as one of intense sedation, relaxation, anxiety suppression and decreased inhibition. It contains a large number of typical depressant cognitive effects.
The most prominent of these cognitive effects generally include:
- Amnesia
- Cognitive euphoria - The euphoria felt on temazepam is significantly stronger than that felt on other benzodiazepines such as alprazolam.
- Anxiety suppression
- Thought deceleration
- Analysis suppression
- Disinhibition
- Compulsive redosing
- Emotion suppression - Although this compound primarily suppresses anxiety, it also dulls other emotions in a manner which is distinct but less intensive than that of antipsychotics.
- Delusions of sobriety - This is the false belief that one is perfectly sober despite obvious evidence to the contrary such as severe cognitive impairment and an inability to fully communicate with others. It most commonly occurs at heavy dosages.
- Memory suppression - Temazepam primarily suppresses short-term memory, resulting in forgetfulness, and/or disorganized behaviors.
- Confusion - At heavy doses, temazepam can cause confusion. This effect is a result of the drug suppressing basic cognitive functions at heavy doses, such as comprehension, memory, and reasoning skills.
- Motivation suppression - Due to temazepam's heavy sedation and lethargy, doing any type of activity that requires moving, or high amounts of effort may be difficult to do on this compound, especially at higher doses.
- Language suppression - Temazepam is known to cause slurred speech and difficulty communicating words in a clear fashion.
- Dream potentiation
Experience reports
There are currently no anecdotal reports which describe the effects of this compound within our experience index. Additional experience reports can be found here:
Toxicity and harm potential
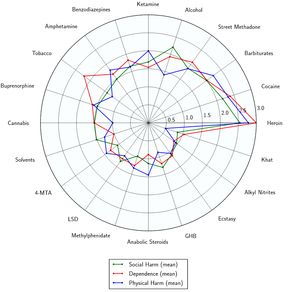
Temazepam had the highest rate of drug intoxication, including overdose, among common benzodiazepines in cases with and without combination with alcohol in a 1985 study.[16] Temazepam and nitrazepam were the two benzodiazepines most commonly detected in overdose-related deaths in an Australian study of drug deaths.[17] A 1993 British study found temazepam to have the highest number of deaths per million prescriptions among medications commonly prescribed in the 1980s (11.9, versus 5.9 for benzodiazepines overall, taken with or without alcohol). Temazepam has a fatal toxicity index (FTI) that is higher than some tricyclic antidepressants, and has also caused overdose and death without combining it with other CNS depressants, which is almost unheard of amongst the benzodiazepine class of drugs.[18]
Another 1995 Australian study of patients admitted to hospital after benzodiazepine overdose corroborated these results, and found temazepam overdose much more likely to lead to coma than other benzodiazepines (odds ratio 1.86). The authors noted several factors, such as differences in potency, receptor affinity, and rate of absorption between benzodiazepines, could explain this higher toxicity. Although benzodiazepines have a high therapeutic index, temazepam is one of the more dangerous of this class of drugs. The combination of alcohol or opioids and temazepam makes death by alcohol poisoning more likely.
Still, temazepam has a low toxicity relative to dose.[4] However, it is potentially lethal when mixed with depressants like alcohol or opioids.
It is strongly recommended that one use harm reduction practices when using this substances. These include volumetric liquid dosing.
Dependence and abuse potential
 |
This toxicity and harm potential section is a stub. As a result, it may contain incomplete or even dangerously wrong information! You can help by expanding upon or correcting it. |
Temazepam is extremely physically and psychologically addictive. Temazepam is considered to be significantly more addictive than other benzodiazepines.
Tolerance will develop to the sedative-hypnotic effects within a couple of days of continuous use.[19] After cessation, the tolerance returns to baseline in 7-14 days. However, in certain cases this may take significantly longer in a manner which is proportional to the duration and intensity of one's long-term usage.
Withdrawal symptoms or rebound symptoms may occur after ceasing usage abruptly following a few weeks or longer of steady dosing, and may necessitate a gradual dose reduction.[20][21] For more information on tapering from benzodiazepines in a controlled manner, please see this guide.
Benzodiazepine discontinuation is notoriously difficult; it is potentially life-threatening for individuals using regularly to discontinue use without tapering their dose over a period of weeks. There is an increased risk of hypertension, seizures, and death.[6] Drugs which lower the seizure threshold such as tramadol should be avoided during withdrawal.
Temazepam presents cross-tolerance with all benzodiazepines, meaning that after its consumption all benzodiazepines will have a reduced effect. Temazepam also has cross-tolerance with all barbiturates, meaning that after consumption, all barbiturates will have a diminished effect.
Overdose
Benzodiazepine overdose may occur when a benzodiazepine is taken in extremely heavy quantities or concurrently with other depressants. This is particularly dangerous with other GABAergic depressants such as barbiturates and alcohol since they work in a similar fashion, but bind to distinct allosteric sites on the GABAA receptor, thus their effects potentiate one another. Benzodiazepines increase the frequency in which the chlorine ion pore opens on the GABAA receptor while barbiturates increase the duration in which they are open, meaning when both are consumed, the ion pore will open more frequently and stay open longer[22]. Benzodiazepine overdose is a medical emergency that may lead to a coma, permanent brain injury or death if not treated promptly and properly.
Symptoms of a benzodiazepine overdose may include severe thought deceleration, slurred speech, confusion, delusions, respiratory depression, coma or death. Benzodiazepine overdoses may be treated effectively in a hospital environment, with generally favorable outcomes. Benzodiazepine overdoses are sometimes treated with flumazenil, a GABAA antagonist[23], however care is primarily supportive in nature.
Dangerous interactions
Although many drugs are safe on their own, they can become dangerous and even life-threatening when combined with other substances. The list below contains some common potentially dangerous combinations, but may not include all of them. Certain combinations may be safe in low doses of each but still increase the potential risk of death. Independent research should always be done to ensure that a combination of two or more substances is safe before consumption.
- Depressants (1,4-Butanediol, 2-methyl-2-butanol, alcohol, barbiturates, GHB/GBL, methaqualone, opioids) - This combination can result in dangerous or even fatal levels of respiratory depression. These substances potentiate the muscle relaxation, sedation and amnesia caused by one another and can lead to unexpected loss of consciousness at high doses. There is also an increased risk of vomiting during unconsciousness and death from the resulting suffocation. If this occurs, users should attempt to fall asleep in the recovery position or have a friend move them into it.
- Dissociatives - This combination can result in an increased risk of vomiting during unconsciousness and death from the resulting suffocation. If this occurs, users should attempt to fall asleep in the recovery position or have a friend move them into it.
- Stimulants - It is dangerous to combine benzodiazepines with stimulants due to the risk of excessive intoxication. Stimulants decrease the sedative effect of benzodiazepines, which is the main factor most people consider when determining their level of intoxication. Once the stimulant wears off, the effects of benzodiazepines will be significantly increased, leading to intensified disinhibition as well as other effects. If combined, one should strictly limit themselves to only dosing a certain amount of benzodiazepines per hour. This combination can also potentially result in severe dehydration if hydration is not monitored.
Legal status
Internationally, Temazepam is included under the United Nations Convention on Psychotropic Substances as a Schedule IV substance.[24]
- Australia: Temazepam is listed in Schedule 4, making it a prescription only medicine.[citation needed]
- Austria: Temazepam is legal for medical use under the AMG (Arzneimittelgesetz Österreich) and illegal when sold or possessed without a prescription under the SMG (Suchtmittelgesetz Österreich).[citation needed]
- Canada: Temazepam is listed on the CSDA in Schedule IV.[25] It requires a prescription.
- Germany: Temazepam is controlled under Anlage III BtMG (Narcotics Act, Schedule III)[26] as of August 1, 1986.[27] It can only be prescribed on a narcotic prescription form, except preparations which contain up to 20 mg temazepam in each dosage form.[26]
- Hong Kong: Temazepam is listed in Schedule 1.[citation needed]
- Ireland: Temazepam is listed in Schedule 3 of the Misuse of Drugs Act 1977 as of November 22, 1993.[28]
- The Netherlands: In the Netherlands, temazepam is a List 2 substance of the Opium Law and is available with a prescription.[citation needed]
- Portugal: Temazepam is a Schedule IV controlled substance.[29]
- Russia: Temazepam is a Schedule III controlled substance since 2013.[30]
- South Africa: Temazepam is a Schedule 5 controlled substance.[citation needed]
- Sweden: Temazepam is a prescription drug in List IV (Schedule 4) under the Narcotics Drugs Act (1968).[31]
- Switzerland: Temazepam is a controlled substance specifically named under Verzeichnis B. Medicinal use is permitted.[32]
- Thailand: Temazepam is listed in Schedule II of the Psychotropic Substances Act.[citation needed]
- United Kingdom: Temazepam is a Class C controlled substance under the Misuse of Drugs Act 1971.[33]. Medical professions must follow specific instructions for the prescribing and disposal of temazepam.
- United States: Temazepam is a prescription medication assigned to Schedule IV of the Controlled Substances Act by the DEA.[34] Many states in the United States require that individuals have specially encoded prescriptions for temazepam.
See also
External links
References
- ↑ Risks of Combining Depressants - TripSit
- ↑ 2.0 2.1 2.2 Collins SR. "Pharmacology and the Nursing Process (2015)". Elsevier Health Sciences. ISBN 9780323358286.
- ↑ 3.0 3.1 3.2 3.3 Müller FO, Van Dyk M, Hundt HK, et al. (1987). "Pharmacokinetics of temazepam after day-time and night-time oral administration". Eur. J. Clin. Pharmacol. 33 (2): 211–4. doi:10.1007/BF00544571. PMID 2891534.
- ↑ 4.0 4.1 Mandrioli, R., Mercolini, L., Raggi, M. A. (October 2008). "Benzodiazepine metabolism: an analytical perspective". Current Drug Metabolism. 9 (8): 827–844. doi:10.2174/138920008786049258. ISSN 1389-2002.
- ↑ "RESTORIL® Novartis Temazepam Hypnotic". Pharmaceutical Information. RxMed.
- ↑ 6.0 6.1 Lann, M. A., Molina, D. K. (June 2009). "A fatal case of benzodiazepine withdrawal". The American Journal of Forensic Medicine and Pathology. 30 (2): 177–179. doi:10.1097/PAF.0b013e3181875aa0. ISSN 1533-404X.
- ↑ Kahan, M., Wilson, L., Mailis-Gagnon, A., Srivastava, A. (November 2011). "Canadian guideline for safe and effective use of opioids for chronic noncancer pain. Appendix B-6: Benzodiazepine Tapering". Canadian Family Physician. 57 (11): 1269–1276. ISSN 0008-350X.
- ↑ Haefely, W. (29 June 1984). "Benzodiazepine interactions with GABA receptors". Neuroscience Letters. 47 (3): 201–206. doi:10.1016/0304-3940(84)90514-7. ISSN 0304-3940.
- ↑ McLean, M. J., Macdonald, R. L. (February 1988). "Benzodiazepines, but not beta carbolines, limit high frequency repetitive firing of action potentials of spinal cord neurons in cell culture". The Journal of Pharmacology and Experimental Therapeutics. 244 (2): 789–795. ISSN 0022-3565.
- ↑ Welt, T., Engelmann, M., Renner, U., Erhardt, A., Müller, M. B., Landgraf, R., Holsboer, F., Keck, M. E. (December 2006). "Temazepam triggers the release of vasopressin into the rat hypothalamic paraventricular nucleus: novel insight into benzodiazepine action on hypothalamic-pituitary-adrenocortical system activity during stress". Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 31 (12): 2573–2579. doi:10.1038/sj.npp.1301006. ISSN 0893-133X.
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/18922233 | Saïas T, Gallarda T | Paradoxical aggressive reactions to benzodiazepine use: a review
- ↑ Paton, C. (December 2002). "Benzodiazepines and disinhibition: a review". Psychiatric Bulletin. 26 (12): 460–462. doi:10.1192/pb.26.12.460. ISSN 0955-6036.
- ↑ Bond, A. J. (1 January 1998). "Drug- Induced Behavioural Disinhibition". CNS Drugs. 9 (1): 41–57. doi:10.2165/00023210-199809010-00005. ISSN 1179-1934.
- ↑ Drummer, O. H. (February 2002). "Benzodiazepines - Effects on Human Performance and Behavior". Forensic Science Review. 14 (1–2): 1–14. ISSN 1042-7201.
- ↑ Nutt, D., King, L. A., Saulsbury, W., Blakemore, C. (24 March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". The Lancet. 369 (9566): 1047–1053. doi:10.1016/S0140-6736(07)60464-4. ISSN 0140-6736.
- ↑ Buckley N. A.; Dawson, A. H.; Whyte, I. M.; O'Connell, D. L. (1995). "Relative toxicity of benzodiazepines in overdose". BMJ. 310 (6974): 219–221. doi:10.1136/bmj.310.6974.219. PMC 2548618
 . PMID 7866122.
. PMID 7866122.
- ↑ Drummer OH; Ranson DL (December 1996). "Sudden death and benzodiazepines". Am J Forensic Med Pathol. 17 (4): 336–42. doi:10.1097/00000433-199612000-00012. PMID 8947361.
- ↑ Serfaty M, Masterton G (1993). "Fatal poisonings attributed to benzodiazepines in Britain during the 1980s". Br J Psychiatry. 163 (3): 386–93. doi:10.1192/bjp.163.3.386. PMID 8104653.
- ↑ Janicak, P. G., Marder, S. R., Pavuluri, M. N. (25 October 2010). Principles and Practice of Psychopharmacotherapy. Lippincott Williams & Wilkins. ISBN 9781605475653.
- ↑ Verster, J. C., Volkerts, E. R. (7 June 2006). "Clinical Pharmacology, Clinical Efficacy, and Behavioral Toxicity of Alprazolam: A Review of the Literature". CNS Drug Reviews. 10 (1): 45–76. doi:10.1111/j.1527-3458.2004.tb00003.x. ISSN 1080-563X.
- ↑ Galanter, M., Kleber, H. D. (2008). The American Psychiatric Publishing Textbook of Substance Abuse Treatment. American Psychiatric Pub. ISBN 9781585622764.
- ↑ Twyman, R. E., Rogers, C. J., Macdonald, R. L. (March 1989). "Differential regulation of gamma-aminobutyric acid receptor channels by diazepam and phenobarbital". Annals of Neurology. 25 (3): 213–220. doi:10.1002/ana.410250302. ISSN 0364-5134.
- ↑ Hoffman, E. J., Warren, E. W. (September 1993). "Flumazenil: a benzodiazepine antagonist". Clinical Pharmacy. 12 (9): 641–656; quiz 699–701. ISSN 0278-2677.
- ↑ List of Psychotropic Substances under International Control | http://www.incb.org/documents/Psychotropics/green_lists/Green_list_ENG_2014_85222_GHB.pdf
- ↑ "Controlled Drugs and Substances Act - SCHEDULE IV". Government of Canada. Retrieved December 29, 2019.
- ↑ 26.0 26.1 "Anlage III BtMG" (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 26, 2019.
- ↑ "Zweite Verordnung zur Änderung betäubungsmittelrechtlicher Vorschriften" (PDF). Bundesgesetzblatt Jahrgang 1986 Teil I Nr. 36 (in German). Bundesanzeiger Verlag. July 29, 1986. Retrieved December 26, 2019.
- ↑ "S.I. No. 342/1993 - Misuse of Drugs (Amendment) Regulations, 1993". Government of Ireland. Retrieved December 29, 2019.
- ↑ "Decreto-Lei n.º 15/93, de 22 de Janeiro" (PDF). Infarmed. Archived from the original (PDF) on April 25, 2010. Retrieved December 29, 2019.
- ↑ Постановление Правительства РФ от 04.02.2013 N 78 “О внесении изменений в некоторые акты Правительства Российской Федерации” - КонсультантПлюс
- ↑ "Läkemedelsverkets föreskrifter (LVFS 2011:10) om förteckningar över narkotika" [Medical Products Agency on the lists of drugs] | http://www.lakemedelsverket.se/upload/lvfs/konsoliderade/LVFS_2011_10_konsoliderad_tom_2012_6.pdf
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
- ↑ Participation, E., Misuse of Drugs Act 1971
- ↑ DEA, Drug Scheduling | http://www.deadiversion.usdoj.gov/schedules/index.html