1,4-Butanediol

Fatal overdose may occur when GABAergic substances are combined with other depressants such as opiates, benzodiazepines, barbiturates, gabapentinoids, thienodiazepines or alcohol.[1]
It is strongly discouraged to combine these substances, particularly in common to heavy doses.
| Summary sheet: 1,4-Butanediol |
| 1,4-Butanediol | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||
| Common names | 1,4-Butanediol, 1,4-B, BD, BDO, One Comma Four, One Four Bee, Butylene Glycol, or One Four B-D-O | ||||||||||||||||||||||||||||||
| Systematic name | Butane-1,4-diol | ||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||
| Psychoactive class | Depressant | ||||||||||||||||||||||||||||||
| Chemical class | Alkanediol | ||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||
| Stimulants | |||||||||||||||||||||||||||||||
| Depressants | |||||||||||||||||||||||||||||||
| Dissociatives | |||||||||||||||||||||||||||||||
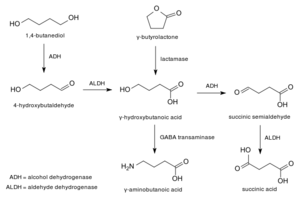
1,4-Butanediol (also known as 1,4-B, BDO, BD or 1,4-BD) is a depressant substance and a prodrug for GHB. It occurs as a thick, colorless liquid or solid depending on storage temperature (melting point of 20 ℃), and has a distinct bitter-sweet taste.[3] It is used as a recreational intoxicant with effects similar to alcohol and GHB.[4] 1 ml of 1,4-butanediol is equivalent to 1.4 g of Na-GHB.
1,4-Butanediol is used industrially as a solvent and in the manufacture of some types of plastics, elastic fibers and polyurethanes. In organic chemistry, it is used for the synthesis of γ-butyrolactone (GBL).[5]
1,4-Butanediol, as well as GBL, will dissolve most types of plastic over time.[6] For this reason, it is recommended to only transport and store the drug using a glass container, standard gelatin capsules (not vegetarian), or high-density polyethylene plastic (also known as #2 recycled plastic). To check the type of plastic used on a bottle, one can look at the bottom for a number in the triangle-shaped recycling label.
Chemistry
1,4-Butanediol is classified as a subclass of alcoholic compounds called diols. Diols are named for having two alcohol (OH-) substitutions in their structure. 1,4-Butanediol is comprised of a butane chain of four carbon groups with an alcohol group bound to each terminal carbon of this chain. 1,4-Butanediol is named for these alcohol substitutions, which are located at R1 and R4. These alcohol substitutions make 1,4-Butanediol a polar liquid, which explains its good solubility in water.
Physically, it is a hygroscopic colorless oily liquid with a barely noticeable characteristic odor. Unlike GHB, 1,4-Butanediol has a distinct taste, described as being repulsive, plastic-like and chemical.
Pharmacology
1,4-Butanediol is not active in its own right; its mechanism of action stems from its identity as a prodrug of GHB.[7]
It is converted into GHB in the liver by the enzymes alcohol dehydrogenase and aldehyde dehydrogenase, which is the same enzyme as alcohol.[8] 1,4-Butanediol is first converted into 4-hydroxybutaldehyde in the liver and is released into the bloodstream before returning to the liver to convert into GHB. This process results in a much more delayed onset than GBL or GHB.[9] 4-hydroxybutaldehyde, even though it is an potentially toxic metabolite, no toxicity identified. Possibly because the very fast metabolism to GHB.[10]
The differing levels of dehydrogenase enzymes can vary between individuals, meaning that, like alcohol, effects can differ greatly between users. In many, this manifests as a slow onset of effects and a higher rate of aldehyde collecting in the bloodstream.Template:Cn Because of these pharmacokinetic differences, 1,4-butanediol tends to be less potent and with a slower onset than GHB but has a longer duration; the related compound GBL tends to be slightly more potent and faster to take effect but more short-acting than GHB.
Subjective effects
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Stimulation and Sedation - At lower dosages, 1,4-Butanediol is physically stimulating, encouraging movement and wakefulness. At higher dosages, however, it becomes physically sedating, encouraging sleep and lethargy.
- Respiratory depression - In cases of 1,4-butanediol overdoses, many reportedly experience an abnormal pattern of breathing characterized by progressively deeper and sometimes faster breathing, followed by a gradual decrease that results in a temporary stop in breathing called an apnea.
- Euphoria
- Nausea - This effect is more common with 1,4-butanediol than GHB.
- Stomach cramps
- Motor control loss
- Dizziness
- Dehydration - This effect is related to the hygroscopic nature of 1,4-Butanediol.
- Muscle cramps
- Optical sliding
- Increased salivation
Cognitive effects 
-
- Anxiety suppression
- Disinhibition
- Cognitive euphoria
- Empathy, affection, and sociability enhancement - Unlike alcohol which merely increases sociability through disinhibition, 1,4-butanediol presents strong entactogenic effects which are prominent and well defined although weaker than that of MDMA.
- Analysis suppression
- Thought deceleration
- Amnesia
- Suggestibility enhancement
- Increased libido
- Increased music appreciation
Experience reports
Anecdotal reports which describe the effects of this compound within our experience index include:
Additional experience reports can be found here
Toxicity and harm potential
1,4-Butanediol is not active in its own right; its mechanism of action stems from its identity as a prodrug of GHB, meaning that it is rapidly converted into GHB in the body.
GHB is considered to be a safe and non-toxic substance when used responsibly or medically. The LD50 is above the active dosage, and there is no danger of acute toxicity when this compound is taken at appropriate dosages. However, it can be dangerous when used as a recreational drug or abused. There have been many negative reports from recreational users who have overdosed, combined GHB with alcohol or other drugs, or accidentally dosed themselves unexpectedly.[12]
One publication has investigated 226 deaths attributed to GHB.[13] Seventy-one deaths (34%) were caused by GHB alone while the other deaths were from respiratory depression caused by interaction with alcohol or other drugs.
To avoid a possible overdose of GHB/1,4-Butanediol, it is important to start with a low dose and work your way up slowly by increasing the dosage in small increments as the exact toxic dosage is unknown.
Accidental ingestions of 1,4-Butanediol have also occurred due to inadequate storage methods. If 1,4-Butanediol is put into a clear liquid, glass, or bottle, it can be easily mistaken for water. It is recommended to clearly label your 1,4-Butanediol in writing and dye the liquid with blue food coloring so it no longer resembles a drinkable beverage. It is also recommended to store your 1,4-Butanediol in a container that no one would drink out of.
It is strongly recommended that one use harm reduction practices when using this drug.
Neurotoxicity
In multiple studies, GHB has been found to impair spatial memory, working memory, learning and memory in rats with chronic administration.[14][15][16][17] These effects are associated with decreased NMDA receptor expression in the cerebral cortex and possibly other areas as well.[18]
One study found that repeated administration of GHB to rats for 15 days drastically reduced the number of neurons and non-neuronal cells within the hippocampus and in the prefrontal cortex. With doses of 10 mg/kg of GHB, they were decreased by 61% in the hippocampus region and 32% in the prefrontal cortex, and with 100 mg/kg, they were decreased by 38% and 9%, respectively. This paper demonstrates contradicting effects on neuronal loss, with lower doses (10 mg/kg) producing the most neurotoxicity, and higher doses (100 mg/kg) producing less.
Tolerance and addiction potential
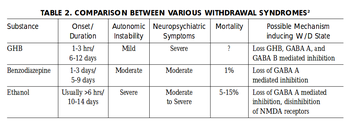
GHB/1,4-Butanediol is moderately physically and psychologically addictive. The frequent use of GHB/1,4-Butanediol can cause withdrawal symptoms similar to those caused by other depressants such as alcohol and benzodiazepines if abruptly discontinued.[20][21] These symptoms seem to depend on the dosage and the length of time the drug was used for. Light to moderate users often experience anxiety, insomnia, sleep-related problems, and tremors whereas heavy use can cause severe withdrawal symptoms like delirium, psychosis, and hallucinations.[22][19]
Although there have been reported fatalities due to GHB/1,4-Butanediol withdrawal, reports are inconclusive and further research is needed.[23]
Tolerance will develop to the sedative-hypnotic effects within several weeks of continuous use. After cessation, the tolerance returns to baseline in 7 - 14 days. Withdrawal symptoms or rebound symptoms may occur after ceasing usage abruptly following a few weeks or longer of steady dosing, and may necessitate a gradual dose reduction.
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- Depressants (1,4-Butanediol, 2M2B, alcohol, benzodiazepines, barbiturates, GHB/GBL, methaqualone, opioids) - This combination potentiates the muscle relaxation, amnesia, sedation, and respiratory depression caused by one another. At higher doses, it can lead to a sudden, unexpected loss of consciousness along with a dangerous amount of depressed respiration. There is also an increased risk of suffocating on one's vomit while unconscious. If nausea or vomiting occurs before a loss of consciousness, users should attempt to fall asleep in the recovery position or have a friend move them into it.
- Dissociatives - This combination can unpredictably potentiate the amnesia, sedation, motor control loss and delusions that can be caused by each other. It may also result in a sudden loss of consciousness accompanied by a dangerous degree of respiratory depression. If nausea or vomiting occurs before consciousness is lost, users should attempt to fall asleep in the recovery position or have a friend move them into it.
- Stimulants - Stimulants mask the sedative effect of depressants, which is the main factor most people use to gauge their level of intoxication. Once the stimulant effects wear off, the effects of the depressant will significantly increase, leading to intensified disinhibition, motor control loss, and dangerous black-out states. This combination can also potentially result in severe dehydration if one's fluid intake is not closely monitored. If choosing to combine these substances, one should strictly limit themselves to a pre-set schedule of dosing only a certain amount per hour until a maximum threshold has been reached.
Legal status
- United States: While 1,4-butanediol is not currently scheduled federally in the United States, a number of states have classified it as a controlled substance. Additionally, individuals have been prosecuted for this substance under the Federal Analogue Act as being substantially similar to GHB.[24] A federal district court in Chicago ruled that 1,4-butanediol could not be considered an analogue of GHB under federal law, and the Seventh Circuit Court of Appeals upheld that ruling.[25]
- United Kingdom: In the United Kingdom, 1,4-butanediol was scheduled in December 2009 (along with another GHB precursor, gamma-butyrolactone) as a Class C controlled substance.
- Germany: 1,4-butanediol is not a controlled substance under the BtMG (Narcotics Act) or the NpSG (New Psychoactive Substances Act).[26][27] It is legal, as long as it is not sold for human consumption, according to §2 AMG.[28]
- Canada: It is controlled as a Schedule VI precursor in Canada.
- Switzerland: 1,4-Butanediol is a controlled substance specifically named under Verzeichnis E.[29]
See also
External links
References
- ↑ Risks of Combining Depressants - TripSit
- ↑ WHO report on 1,4-butanediol | https://www.who.int/medicines/areas/quality_safety/4_4_Review.pdf
- ↑ PubChem, 1,4-Butanediol
- ↑ Zvosec, D. L., Smith, S. W., McCutcheon, J. R., Spillane, J., Hall, B. J., Peacock, E. A. (11 January 2001). "Adverse Events, Including Death, Associated with the Use of 1,4-Butanediol". New England Journal of Medicine. 344 (2): 87–94. doi:10.1056/NEJM200101113440202. ISSN 0028-4793.
- ↑ Zhao, J., Hartwig, J. F. (1 May 2005). "Acceptorless, Neat, Ruthenium-Catalyzed Dehydrogenative Cyclization of Diols to Lactones". Organometallics. 24 (10): 2441–2446. doi:10.1021/om048983m. ISSN 0276-7333.
- ↑ Erowid 1,4-butanediol Vault : Storage : 14b and GBL May Dissolve Some Plastics, 2001
- ↑ Carter, L. P., Koek, W., France, C. P. (November 2006). "Lack of effects of GHB precursors GBL and 1,4-BD following i.c.v. administration in rats: GBL and 1,4-BD after i.p. and i.c.v. administration". European Journal of Neuroscience. 24 (9): 2595–2600. doi:10.1111/j.1460-9568.2006.05146.x. ISSN 0953-816X.
- ↑ "Gamma-Hydroxybutyrate Toxicity: Practice Essentials, Background, Pathophysiology". 12 May 2022.
- ↑ Burk, M. J., Van Dien, S. J., Burgard, A. P., Niu, W., United States Patent: 8067214 - Compositions and methods for the biosynthesis of 1,4-butanediol and its precursors
- ↑ Irwin, R. D. (February 2006). "A review of evidence leading to the prediction that 1,4-butanediol is not a carcinogen". Journal of applied toxicology: JAT. 26 (1): 72–80. doi:10.1002/jat.1110. ISSN 0260-437X.
- ↑ Nutt, D., King, L. A., Saulsbury, W., Blakemore, C. (24 March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". The Lancet. 369 (9566): 1047–1053. doi:10.1016/S0140-6736(07)60464-4. ISSN 0140-6736.
- ↑ GHB - Erowid Exp - “GHB Overdoses & Poisonings”
- ↑ Zvosec, D. L., Smith, S. W., Porrata, T., Strobl, A. Q., Dyer, J. E. (March 2011). "Case series of 226 γ-hydroxybutyrate-associated deaths: lethal toxicity and trauma". The American Journal of Emergency Medicine. 29 (3): 319–332. doi:10.1016/j.ajem.2009.11.008. ISSN 1532-8171.
- ↑ Sircar, R., Basak, A. (1 December 2004). "Adolescent γ-hydroxybutyric acid exposure decreases cortical N-methyl-d-aspartate receptor and impairs spatial learning". Pharmacology Biochemistry and Behavior. 79 (4): 701–708. doi:10.1016/j.pbb.2004.09.022. ISSN 0091-3057.
- ↑ García, F. B., Pedraza, C., Arias, J. L., Navarro, J. F. (August 2006). "[Effects of subchronic administration of gammahydroxybutyrate (GHB) on spatial working memory in rats]". Psicothema. 18 (3): 519–524. ISSN 0214-9915.
- ↑ Sircar, R., Basak, A., Sircar, D. (October 2008). "γ-Hydroxybutyric Acid-Induced Cognitive Deficits in the Female Adolescent Rat". Annals of the New York Academy of Sciences. 1139 (1): 386–389. doi:10.1196/annals.1432.044. ISSN 0077-8923.
- ↑ Pedraza, C., García, F. B., Navarro, J. F. (October 2009). "Neurotoxic effects induced by gammahydroxybutyric acid (GHB) in male rats". The International Journal of Neuropsychopharmacology. 12 (09): 1165. doi:10.1017/S1461145709000157. ISSN 1461-1457.
- ↑ Sircar, R., Basak, A. (December 2004). "Adolescent gamma-hydroxybutyric acid exposure decreases cortical N-methyl-D-aspartate receptor and impairs spatial learning". Pharmacology, Biochemistry, and Behavior. 79 (4): 701–708. doi:10.1016/j.pbb.2004.09.022. ISSN 0091-3057.
- ↑ 19.0 19.1 GHB Withdrawal Syndrome | Texas Commission on Alcohol and Drug Abuse | https://www.erowid.org/chemicals/ghb/ghb_addiction2.pdf
- ↑ Kim, S. Y., Barker, J. C., Anderson, I. B., Dyer, J. E., Earnest, G., Blanc, P. D. (2008). "Systematic Assessment of Gamma Hydroxybutyrate (GHB) Effects During and After Acute Intoxication". The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 17 (4): 312–318. doi:10.1080/10550490802138988. ISSN 1055-0496.
- ↑ Carter, L. P., Pardi, D., Gorsline, J., Griffiths, R. R. (1 September 2009). "Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem®): differences in characteristics and misuse". Drug and alcohol dependence. 104 (1–2): 1–10. doi:10.1016/j.drugalcdep.2009.04.012. ISSN 0376-8716.
- ↑ Dyer, J. E., Roth, B., Hyma, B. A. (February 2001). "Gamma-hydroxybutyrate withdrawal syndrome". Annals of Emergency Medicine. 37 (2): 147–153. doi:10.1067/mem.2001.112985. ISSN 0196-0644.
- ↑ Galloway, G. P., Frederick, S. L., Staggers, F. E., Gonzales, M., Stalcup, S. A., Smith, D. E. (January 1997). "Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence". Addiction. 92 (1): 89–96. doi:10.1111/j.1360-0443.1997.tb03640.x. ISSN 0965-2140.
- ↑ United States of America, Appellee, v. Thomas William Washam, Appellant, 312 F.3d 926 (8th Cir. 2003)
- ↑ United States v. Turcotte, 405 F.3d 515 (7th Cir. 2005) "With specific regard to 1,4-butanediol, the jury has returned a special verdict which states that 1,4-butanediol is not a Schedule I drug analogue, because 1,4-butanediol's chemical structure is not significantly similar to the chemical structure of GHB.
- ↑ BtMG | http://www.gesetze-im-internet.de/btmg_1981/BtMG.pdf
- ↑ NpSG | https://www.gesetze-im-internet.de/npsg/NpSG.pdf
- ↑ § 2 AMG - Einzelnorm
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.