Adrafinil
| Summary sheet: Adrafinil |
| Adrafinil | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||
| Common names | Adrafinil, Olmifon | ||||||||||||||||||||||||||||||||
| Substitutive name | Adrafinil | ||||||||||||||||||||||||||||||||
| Systematic name | (±)-2-Benzhydrylsulfinylethanehydroxamic acid | ||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||
| Psychoactive class | Stimulant / Eugeroic | ||||||||||||||||||||||||||||||||
| Chemical class | Benzhydryl | ||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||
Adrafinil (also known as Olmifon) is a prodrug for modafinil - a wakefulness-promoting agent (eugeroic) with nootropic effects. Adrafinil is metabolized in the liver to produce modafinil. Both are stimulants with no amphetamine-like effects. Safety information on adrafinil is lacking because modafinil is often used instead, for treatment of narcolepsy and excessive daytime sleepiness. Long-term supplementation of adrafinil is not advised, since adrafinil is metabolized into modafinil in the liver, and may stress the liver through elevated liver enzymes with prolonged use.
Chemistry
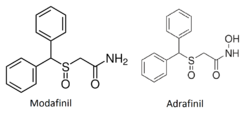
Adrafinil is very structurally similar to its close chemical cousin and bioactive metabolite, modafinil. The only structural difference is that terminal amide hydroxyl group of adrafinil ((diphenylmethyl)sulfinyl-2 acetohydroxamic acid) is lacking in modafinil (diphenylmethyl)sulfinyl-2 acetamide).[1]
Pharmacology
The mechanism of adrafinil appears to rely on postsynaptic α-adrenergic activity since an increase in locomotion caused by adrafinil is blocked by prazosin (α1 antagonist), yohimbine (α2 antagonist), or phenoxybenzamine (mixed α-antagonist).[2] These increases through adrenergic neurotransmission are thought to be responsible for adrafinil's energetic properties.
Orally ingested adrafinil may undergo one of two metabolic results. The main metabolite is the active modafinil, which is itself metabolized to inactive modafinilic acid and modafinil sulfone. Adrafinil, however, can also be metabolized directly to inactive modafinilic acid without conversion to modafinil. [3] This may account for its lower potency.
Subjective effects
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Experience reports
Anecdotal reports which describe the effects of this compound within our experience index include:
Additional experience reports can be found here:
Toxicity and harm potential
The long-term safety and effectiveness of modafinil (adrafinil's active component) as a drug of regular usage have not been determined.[4] Anecdotal reports from people who have tried modafinil within the community suggest that there do not seem to be any negative health effects attributed to simply trying this substance at low to moderate doses by itself and using it sparingly (but nothing can be completely guaranteed).
Adrafinil is more harmful than modafinil due to slight hepatotoxicity, as adrafinil must be processed by the liver. Stomach pain, skin irritation, anxiety, and elevated liver enzymes may occur with prolonged use and have been associated with Adrafinil, specifically.[5]
It is strongly recommended that one use harm reduction practices when using this substance.
Tolerance and addiction potential
The chronic use of adrafinil can be considered as not addictive with a low potential for abuse. It does not seem to be capable of causing psychological dependence among most users.[citation needed]
Tolerance to many of the effects of adrafinil develops with prolonged and repeated use. This results in users having to administer increasingly large doses to achieve the same effects. After that, it takes about 3 - 7 days for the tolerance to be reduced to half and 1 - 2 weeks to be back at baseline (in the absence of further consumption). Adrafinil may present a cross-tolerance with all benzhydryl nootropics, meaning that after the consumption of adrafinil, all related eugeroic compounds such as armodafinil and modafinil will display a reduced effect.
Legal status
 |
This legality section is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
Unlike modafinil, adrafinil is legal to sell and possess without a prescription in most countries.[citation needed]
See also
External links
References
- ↑ Milgram, N. W., Callahan, H., Siwak, C. (7 June 2006). "Adrafinil: A Novel Vigilance Promoting Agent". CNS Drug Reviews. 5 (3): 193–212. doi:10.1111/j.1527-3458.1999.tb00100.x. ISSN 1080-563X.
- ↑ Duteil, J., Rambert, F. A., Pessonnier, J., Gombert, R., Assous, E. (26 October 1979). "A possibe alpha-adrenergic mechanism for drug (CRL 40028)-induced hyperactivity". European Journal of Pharmacology. 59 (1–2): 121–123. doi:10.1016/0014-2999(79)90033-5. ISSN 0014-2999.
- ↑ Burnat, P., Robles, F., Do, B. (20 March 1998). "High-performance liquid chromatographic determination of modafinil and its two metabolites in human plasma using solid-phase extraction". Journal of Chromatography. B, Biomedical Sciences and Applications. 706 (2): 295–304. doi:10.1016/s0378-4347(97)00550-1. ISSN 1387-2273.
- ↑ Banerjee, D., Vitiello, M. V., Grunstein, R. R. (1 October 2004). "Pharmacotherapy for excessive daytime sleepiness". Sleep Medicine Reviews. 8 (5): 339–354. doi:10.1016/j.smrv.2004.03.002. ISSN 1087-0792.
- ↑ Ballas, C. A., Kim, D., Baldassano, C. F., Hoeh, N. (July 2002). "Modafinil: past, present and future". Expert Review of Neurotherapeutics. 2 (4): 449–457. doi:10.1586/14737175.2.4.449. ISSN 1744-8360.