Heroin

Fatal overdose may occur when opiates are combined with other depressants such as benzodiazepines, barbiturates, gabapentinoids, thienodiazepines, alcohol or other GABAergic substances.[1]
It is strongly discouraged to combine these substances, particularly in common to heavy doses.
| Summary sheet: Heroin |
| Heroin | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common names | Heroin, H, Smack, Junk, Brown | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substitutive name | Diacetylmorphine (Diamorphine) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Systematic name | (5α,6α)-7,8-Didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol diacetate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Psychoactive class | Opioid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical class | Morphinan | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MAOIs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nitrous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PCP | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stimulants | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alcohol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Benzodiazepines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DXM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GHB | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GBL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ketamine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MXE | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tramadol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Grapefruit | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Diacetylmorphine or morphine diacetate (also known as diamorphine and heroin as well as colloquially as H, dope, smack, junk, brown, boy, and others) is a semi-synthetic opioid substance of the morphinan class. It is a derivative of morphine, a natural product of the opium poppy (Papaver somniferum).[citation needed] Heroin is known for its highly addictive properties and it makes up a large portion of the illicit traffic in narcotics.
Heroin was first synthesized from morphine by a British chemist in 1874 and was introduced as a commercial product by the Bayer Company of Germany in 1898.[2] Although the name heroin is a trade name, it has since been widely adopted for all intents and purposes and may describe a recreational depressant that may or may not contain pure diacetylmorphine.
Subjective effects include sedation, pain relief, muscle relaxation, and euphoria.
In its pure form, heroin is active at doses of 5 mg and above; however, the substance is most commonly found in a preparation containing significant impurities. These impurities can result from including processing faults during synthesis, the addition of harmful or benign cutting agents, or the substitution of significantly more potent and dangerous analogous substances such as fentanyl adulterated into the end-product before distribution.[3][4][5][6][7] The heroin found on streets may have a significant variation in purity, but on average is about 5%. This makes accurate dosing particularly difficult.
It is highly advised to use harm reduction practices if using this substance.
History and culture
This History and culture section is a stub. As a result, it may contain incomplete or wrong information. You can help by expanding it. |
Diacetylmorphine was originally synthesized by C.R. Alder Wright in 1874 when attempting to combine morphine with various acids. The synthesis was achieved through boiling anhydrous morphine with morphine alkaloid with acetic anhydride.[2]
Although the name heroin is a traditional trade name for a Bayer product containing diacetylmorphine, the name has since been widely adopted for all intents and purposes and may describe a recreational depressant that may or may not contain pure diacetylmorphine.
Chemistry
Heroin, or morphine diacetate or diacetylmorphine (diamorphine hydrochloride), is an opiate and an ester of morphine. Heroin and other molecules of this class contain a polycyclic core of three benzene rings fused in a zig-zag pattern called a phenanthrene. A fourth nitrogen containing ring is fused to the phenanthrene at R9 and R13, with the nitrogen member looking at R17 of the combined structure. This structure is called morphinan.
Heroin, along with other morphinans, contains an ether bridge between two of its rings, connecting R4 and R5 through an oxygen group. Heroin contains two acetate (CH3COO−) groups bonded to R3 and R6 of its structure, and a methyl group located on the nitrogen atom at R17. On the same ring containing the 6-acetyl group, heroin contains a double bond.
Heroin's chemical structure is closely related to morphine. Heroin is the 3,6-diacetyl derivative of the diol morphine, meaning it contains acetate functional groups at the same locations hydroxy groups are found in morphine (3,6). Heroin is analogous to the other morphinans including dihydrocodeine, codeine, ethylmorphine, hydrocodone, and oxycodone.
Pharmacology
Heroin itself is an inactive drug, but it is metabolized into morphine when inserted into the body.[8] When taken orally, heroin undergoes extensive first-pass metabolism via deacetylation, making it a prodrug for the systemic delivery of morphine.[8] When the drug is injected, however, it avoids this first-pass effect, very rapidly crossing the blood–brain barrier because of the presence of the acetyl groups which render it much more fat-soluble, and thus more potent, than morphine itself.[9]
Once in the brain, it is deacetylated variously into the inactive 3-monoacetylmorphine and the active 6-monoacetylmorphine (6-MAM). 6-MAM is found in some commercially prepared batches of black tar heroin, often found on the west coast of the United States. It is then deacetylated into morphine, which binds to μ-opioid receptors. Heroin itself exhibits relatively low affinity for the μ receptor, suggesting that much of the psychoactive effects of the substance come from the metabolites of heroin rather than the diacetylmorphine itself.[10]
The recreational effects of this compound, including cognitive euphoria and physical euphoria, occur because opioids structurally mimic endogenous endorphins which are naturally produced within the body and are also active on the μ-opioid receptor set in the brain. The way in which synthetic opioids such as heroin structurally mimic these natural endorphins results in their euphoric, pain relief and anxiolytic effects. This is because natural endorphins are responsible for reducing pain, causing sleepiness, and feelings of pleasure. The endorphins can be released in response to pain, strenuous exercise, orgasm, or general excitement.
Subjective effects
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Sedation - This leads to the trademark "nodding off" effect seen in heroin users, where the head falls, the eyes close, and the user slips uncontrollably into momentary unconsciousness.[11] This can lead to falling into microsleeps while sitting or standing up.
- Physical euphoria - The physical euphoria experienced on this substance is extremely intense when compared to other opioids such as codeine or tramadol. The sensation itself can be described as extreme feelings of intense physical comfort, warmth, and all-encompassing bliss.
- Pain relief
- Respiratory depression - Heavy dosages of heroin can result in respiratory depression which leads onto fatal or dangerous levels of anoxia (oxygen deprivation). This occurs because the breathing reflex is suppressed by agonism of µ-opioid receptors proportional to the dosage consumed.
- Appetite suppression
- Dehydration
- Constipation
- Cough suppression
- Decreased libido
- Decreased heart rate
- Difficulty urinating
- Increased perspiration
- Itchiness
- Nausea - This effect can be intense enough to lead to vomiting at high doses. When severe, the nausea and subsequent vomiting can cause dehydration in the user.
- Orgasm suppression
- Pupil constriction
Cognitive effects 
-
- Cognitive euphoria - The cognitive euphoria experienced on this substance is extremely intense when compared to other opioids such as codeine or tramadol. The sensation itself can be described as powerful and overwhelming feelings of emotional bliss, contentment, and happiness.
- Anxiety suppression
- Compulsive redosing
- Dream potentiation
Visual effects 
-
Suppressions
- Double vision - At high doses, the eyes un-focus and re-focus uncontrollably. It appears to be caused by issues in depth perception, as by closing one eye the issue can be temporarily alleviated.
Hallucinatory states
- Internal hallucination - One may experience a state of semi-consciousness and hypnagogia during heavy dosage nodding which results in dream-like states and up to level 3 imagery. This is often accompanied by ill-defined geometry.
Auditory effects 
-
- Auditory acuity suppression
- Auditory distortion - Some users experience rumbling in the ears and/or tinnitus with this substance. Effect may last for long periods after the experience due to the ototoxicity of opioids
Experience reports
Anecdotal reports which describe the effects of this compound within our experience index include:
- Experience:20mg Heroin - The Last Time I Shot Up
- Experience:2955mg 2-Methylmethcathinone + 276mg Diacetylmorphine - Stimulant psychosis ego death nightmare
Additional experience reports can be found here:
Forms
Black tar heroin

Black tar heroin is dangerous to inject
It's not practical to make black tar heroin sterile, for example, by heating a solution with a lighter for a minute. Black tar heroin injection is associated with Clostridium botulinum infection. Prion: "For prion elimination, various recommendations state 121–132 °C (250–270 °F) for 60 minutes or 134 °C (273 °F) for at least 18 minutes."[12] A pressure cooker reach 120 °C at full pressure. However, we don't recommend black tar heroin injection even if you own a pressure cooker with a PSI meter due to lack of safety data.
This form of heroin is diacetylmorphine acetate, a product of heroin production that does not require further acetylation. It differs in texture from powder heroin in that it is black, gooey, viscous, and a texture ranging from quite similar to wet asphalt and a hard rock of material. It is commonly produced in South America and is found on the western coast of the USA.
The actual chemical contents of black tar heroin can vary from the white powder form. Black tar might contain a variable mixture of morphine derivatives, predominantly 6-MAM (6-monoacetylmorphine) which is another result of crude acetylation that occurs in the clandestine manufacturing process.
When injected into any type of tissue, this form of heroin results in an increased risk of wound botulism[13]. Wound botulism can be fatal and leads to amputations and death at a higher rate of black tar heroin users. Because of the consistency of the substance (tar-like), it can pose a greater risk for collapsing, damaging, or infecting veins. This damage to veins leads to a higher chance of subcutaneous and intradermal injection[14] which is not advocated from a harm reduction point of view. For users who do choose to inject this substance, it is advised to follow the procedures found in the safer injection guide.
Toxicity and harm potential

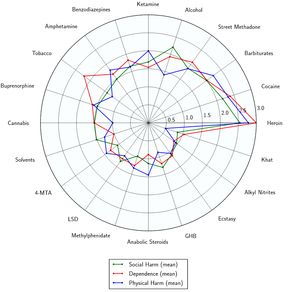
Like many other opioids, unadulterated heroin at appropriate dosages does not cause many long-term complications other than physical and psychological dependence and constipation. Outside of the extremely powerful addiction and physical dependence, the harmful or toxic aspects of opioid usage are exclusively associated with not taking the necessary precautions in regards to its administration, overdosing and using impure heroin products that contain harmful additives.
Heavy dosages of heroin can result in severe respiratory depression which can result in dangerous or even fatal levels of anoxia (oxygen deprivation). This occurs because the breathing reflex is suppressed by agonism of µ-opioid receptors - this effect is proportional to the dosage of the substance consumed.
Due to the nature of the unregulated drug market, illicit heroin is of widely varying and unpredictable purity. A user may prepare what they consider to be a moderate dose while actually taking far more than intended in the event of obtaining a purer product than they are used to, or may be cut unknowingly with more potent and dangerous substances such as fentanyl.[17] Depending on drug interactions and numerous other factors, death from overdose can take anywhere from several minutes to several hours and is a direct result of respiratory depression leading onto anoxia (oxygen deprivation) resulting from the breathing reflex being suppressed by agonism of µ-opioid receptors. Some sources quote the median lethal dose (for an average 75 kg opiate-naive individual) as being between 75 and 600mg.[18]
Heroin can also cause nausea and vomiting; a significant number of deaths attributed to opioid overdose are caused by aspiration of vomit by an unconscious victim. This is when an unconscious or semi-conscious user who is lying on their back vomits into their mouth and unknowingly suffocates. It can be prevented by ensuring that one is lying on their side with their head tilted downwards so that the airways cannot be blocked in the event of vomiting while unconscious (also known as the recovery position).
In case of a suspected or known overdose, it is advised to administer a dose of naloxone intravenously, intramuscularly, or nasally to reverse the effects of opioid agonism[19].
It is strongly recommended that one use harm reduction practices such as volumetric dosing, when using this substance to ensure the administration of the intended dose (especially due to the unusually high risk of an overdose this substance presents.)
Bodily harm
Many opioid drugs, especially oxycodone and heroin, are known to be ototoxic, causing hearing loss, tinnitus and balance issues. In some cases the damage can be irreversible.[20]
Opioids can cause urinary retention, or difficulty urinating and constipation with chronic and repeated use. Some users find relief in blowing hard into their palm or doing squats before using the toilet, as this compresses core muscles onto the bladder.
Dependence and abuse potential
As with other opioids, the chronic use of heroin can be considered extremely addictive with a high potential for abuse and is capable of causing psychological and physical dependence among certain users. When psychological or physical addiction has developed, mental and physical withdrawal symptoms and cravings may occur if a person suddenly stops their usage.
Tolerance to many of the effects of heroin develops with prolonged and repeated use. The rate at which this occurs develops at different rates for different effects, with tolerance to the constipation-inducing effects developing particularly slowly for instance. This results in users having to administer increasingly large doses to achieve the same psychoactive effects of a previously lower dose. After heroin tolerance has developed, it takes about 3 - 7 days for the tolerance to be reduced to half and 1 - 2 weeks to be back at baseline (in the absence of further consumption). Heroin presents cross-tolerance with all other opioids, meaning that after the consumption of heroin all opioids will have a reduced effect.
The risk of fatal heroin overdoses rise sharply after a period of cessation and relapse, largely because of reduced tolerance.[21] When users dose their old doses, they no longer have the physical tolerance to handle the sedative effects of heroin and overdose occurs. To account for this lack of physical tolerance, it is safer to only dose a fraction of one's usual dosage if using after a prolonged period of sobriety. It has also been found that the environment one is in can play a role in tolerance: in one scientific study, rats with the same history of heroin administration were significantly more likely to die after receiving their dose in an environment not associated with the drug in contrast to a familiar environment.[22]
Studies have shown that the subjective cognitive euphoria and physical euphoria of heroin use, which is the reinforcing component of addiction, is proportional in its' intensity to the rate at which the blood level concentrations of the drug increases.[23] Intravenous injection is the fastest route of drug administration, causing blood concentrations to rise the fastest. It is followed by smoking, suppository (anal or vaginal insertion), insufflation (snorting), and ingestion (swallowing).
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- Alcohol - Both substances potentiate the ataxia and sedation caused by the other and can lead to unexpected loss of consciousness at high doses. Place affected patients in the recovery position to prevent vomit aspiration from excess. Memory blackouts are likely
- Stimulants - Stimulants increase respiration rate which allows for a higher dose of opiates than would otherwise be used. If the stimulant wears off first then the opiate may overcome the user and cause respiratory arrest.
- Benzodiazepines - Central nervous system and/or respiratory-depressant effects may be additively or synergistically present. The two substances potentiate each other strongly and unpredictably, very rapidly leading to unconsciousness. While unconscious, vomit aspiration is a risk if not placed in the recovery position blackouts/memory loss likely.
- DXM - Generally considered to be toxic. CNS depression, difficulty breathing, heart issues, and liver toxicity have been observed. Additionally if one takes DXM, their tolerance of opiates goes down slightly, thus causing additional synergistic effects.
- GHB/GBL - The two substances potentiate each other strongly and unpredictably, very rapidly leading to unconsciousness. While unconscious, vomit aspiration is a risk if not placed in the recovery position
- Ketamine - Both substances bring a risk of vomiting and unconsciousness. If the user falls unconscious while under the influence there is a severe risk of vomit aspiration if they are not placed in the recovery position.
- MAOIs - Coadministration of monoamine oxidase inhibitors (MAOIs) with certain opioids has been associated with rare reports of severe adverse reactions. There appear to be two types of interaction, an excitatory and a depressive one. Symptoms of the excitatory reaction may include agitation, headache, diaphoresis, hyperpyrexia, flushing, shivering, myoclonus, rigidity, tremor, diarrhea, hypertension, tachycardia, seizures, and coma. Death has occurred in some cases.
- MXE - MXE can potentiate the effects of opioids but also increases the risk of respiratory depression and organ toxicity.
- Nitrous - Both substances potentiate the ataxia and sedation caused by the other and can lead to unexpected loss of consciousness at high doses. While unconscious, vomit aspiration is a risk if not placed in the recovery position. Memory blackouts are common.
- PCP - PCP may reduce opioid tolerance, increasing the risk of overdose.
- Tramadol - Increased risk of seizures. Tramadol itself is known to induce seizures and it may have additive effects on seizure threshold with other opioids. Central nervous system- and/or respiratory-depressant effects may be additively or synergistically present.
- Grapefruit - While grapefruit is not psychoactive, it may affect the metabolism of certain opioids. Tramadol, oxycodone, and fentanyl are all primarily metabolized by the enzyme CYP3A4, which is potently inhibited by grapefruit juice[24]. This may cause the drug to take longer to clear from the body. it may increase toxicity with repeated doses. Methadone may also be affected[24]. Codeine and hydrocodone are metabolized by CYP2D6. People who are on medicines that inhibit CYP2D6, or that lack the enzyme due to a genetic mutation will not respond to codeine as it can not be metabolized into its active product: morphine.
Legal status
- Australia: Heroin is controlled in Australia, but there is conflicting information about its exact legal status. Heroin was listed in Schedule I of the Narcotic Drugs Act of 1967; however, it is unclear whether the control system has changed since then.[citation needed] Personal quantities under 1 gram have been decriminalized in the Australian Capital Territory (ACT) as of 28 October 2023.[25]
- Austria: Diacetylmorphine is illegal to possess, produce and sell under the SMG (Suchtmittelgesetz Österreich).[citation needed]
- Brazil: Heroin is listed as a controlled substance, making the production, distribution, or possession illegal.[citation needed]
- Canada: Heroin is a Schedule I substance in Canada. However, a unanimous Supreme Court decision in 2011 declared that there is a right under Section 7 of the Charter of Rights and Freedoms to have access to clean injection sites.[26][27]
- Czech Republic: The Czech Republic has decriminalized 1.5g or less of heroin and the punishment is similar in scale to a parking ticket. Sales, production, and larger quantity possession are still crimes.[28]
- Finland: Heroin is a controlled substance, making the production, distribution, and possession illegal without a license.[citation needed]
- Germany: Heroin is controlled under BtMG Anlage I, II and III, making it illegal to manufacture, import, possess, sell, or transfer it without a license. There is an exception for preparations, that are approved for addiction treatment, which can be prescribed on a narcotic prescription form.[29][30][31]
- Italy: Heroin is a Schedule I drug.[32]
- Latvia: Heroin is a Schedule I drug.[33]
- New Zealand: Heroin is Class A in New Zealand.[citation needed]
- Norway: Heroin is Schedule I in Norway and illegal to buy or possess without a special license. There have been some projects to establish needle rooms in Norway by the government where heroin addicts are allowed to get fresh needles for injecting heroin.[citation needed]
- Portugal: The personal use of heroin was decriminalized by Law 30/2000 on July 2001. Possession of less than 1g is not regarded as a criminal offense although the substance is liable to be seized and the possessor can be referred to mandatory treatment. Sale or possession of quantities greater than the personal possession limit are criminal offenses punishable by jail time.[citation needed]
- Russia: Heroin is a Schedule I controlled substance.[34]
- Switzerland: Heroin is a controlled substance specifically named under Verzeichnis D. It is legally available for addicts under an ongoing experiment but is otherwise illegal to possess.[35]
- United Kingdom: Heroin is Schedule II/Class A and is illegal to buy, sell or possess without a license.[citation needed]
- United States: Heroin is Schedule I in the United States. This means it is illegal to manufacture, buy, possess, or distribute without a DEA license.[citation needed]
- Poland: Heroin is illegal to produce, sell and possess under "wykaz środków odurzających i substancji psychotropowych".[citation needed]
See also
External links
- Heroin (Wikipedia)
- Heroin (Erowid Vault)
- Heroin (Isomer Design)
- Heroin (DrugBank)
- Heroin (Drugs-Forum)
Literature
- Schmidt, H., Vormfelde, S. V., Klinder, K., Gundert-Remy, U., Gleiter, C. H., Skopp, G., Aderjan, R. and Fuhr, U. (2002), Affinities of Dihydrocodeine and its Metabolites to Opioid Receptors. Pharmacology & Toxicology, 91: 57–63. https://doi.org/10.1034/j.1600-0773.2002.910203.x
- Pert, C. B., Pasternak, G., & Snyder, S. H. (1973). Opiate Agonists and Antagonists Discriminated by Receptor Binding in Brain. Science, 182(4119), 1359-1361. https://doi.org/10.1126/science.182.4119.1359
- Stefano GB, Ptáček R, Kuželová H, Kream RM (2012). Endogenous morphine: up-to-date review (2011. Folia Biol. (Praha). 58 (2): 49–56. PMID 22578954.
References
- ↑ Risks of Combining Depressants - TripSit
- ↑ 2.0 2.1 Wright, C. R. A. (1874). "XLIX.—On the action of organic acids and their anhydrides on the natural alkaloïds. Part I". J. Chem. Soc. 27 (0): 1031–1043. doi:10.1039/JS8742701031. ISSN 0368-1769.
- ↑ Much of Heroin Supply Adulterated with Fentanyl (PDF), Department of Mental Health and Addiction Services
- ↑ Roberts, J. R. (April 2014). "InFocus: Fentanyl-Laced Heroin". Emergency Medicine News. 36 (4): 13–15. doi:10.1097/01.EEM.0000446051.11866.98. ISSN 1054-0725.
- ↑ Bever, F. (2015), Illicit Version Of Painkiller Fentanyl Makes Heroin Deadlier
- ↑ What You Need to Know About Fentanyl-Laced Heroin | http://www.projectknow.com/what-you-need-to-know-about-fentanyl-laced-heroin/
- ↑ Gorman, R. (2014), Killer white heroin responsible for as many as 100 deaths in the US
- ↑ 8.0 8.1 Sawynok, J. (1 January 1986). "The therapeutic use of heroin: a review of the pharmacological literature". Canadian Journal of Physiology and Pharmacology. 64 (1): 1–6. doi:10.1139/y86-001. ISSN 0008-4212.
- ↑ Klous, M. G., Brink, W. V. den, Ree, J. M. V., Beijnen, J. H. (12 December 2005). "Development of pharmaceutical heroin preparations for medical co-prescription to opioid dependent patients". Drug and Alcohol Dependence. 80 (3): 283–295. doi:10.1016/j.drugalcdep.2005.04.008. ISSN 0376-8716.
- ↑ Inturrisi, C. E., Schultz, M., Shin, S., Umans, J. G., Angel, L., Simon, E. J. (1983). "Evidence from opiate binding studies that heroin acts through its metabolites". Life Sciences. 33 Suppl 1: 773–776. doi:10.1016/0024-3205(83)90616-1. ISSN 0024-3205.
- ↑ Urban Dictionary: nodding off
- ↑ Rutala, WA; Weber, DJ; Society for Healthcare Epidemiology of, America (February 2010). "Guideline for disinfection and sterilization of prion-contaminated medical instruments". Infection control and hospital epidemiology. 31 (2): 107–17. doi:10.1086/650197. PMID 20055640.
- ↑ Passaro, D. J., Werner, S. B., McGee, J., Mac Kenzie, W. R., Vugia, D. J. (18 March 1998). "Wound Botulism Associated With Black Tar Heroin Among Injecting Drug Users". JAMA. 279 (11): 859–863. doi:10.1001/jama.279.11.859. ISSN 0098-7484.
- ↑ Ciccarone, D., Bourgois, P. (January 2003). "Explaining the Geographical Variation of HIV Among Injection Drug Users in the United States". Substance Use & Misuse. 38 (14): 2049–2063. doi:10.1081/JA-120025125. ISSN 1082-6084.
- ↑ Nutt DJ, King LA, Phillips LD (November 2010). "Drug harms in the UK: a multicriteria decision analysis". Lancet. 376 (9752): 1558–1565. CiteSeerX 10.1.1.690.1283
 . doi:10.1016/S0140-6736(10)61462-6. PMID 21036393. Unknown parameter
. doi:10.1016/S0140-6736(10)61462-6. PMID 21036393. Unknown parameter |s2cid=ignored (help) - ↑ Nutt, D., King, L. A., Saulsbury, W., Blakemore, C. (24 March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". The Lancet. 369 (9566): 1047–1053. doi:10.1016/S0140-6736(07)60464-4. ISSN 0140-6736.
- ↑ Zalkind, S. (2016), “Death pill”: fentanyl disguised as other drugs linked to spike in US overdoses
- ↑ The heroin overdose mystery and other occupational hazards of heroin addiction
- ↑ Drugs.com Naloxone Hydrochloride Page|https://www.drugs.com/monograph/naloxone-hydrochloride.html
- ↑ Opioid-Associated Hearing Loss: A 20-Year Review from the New Jersey Poison Center https://pubmed.ncbi.nlm.nih.gov/32468345/
- ↑ Why Heroin Relapse Often Ends In Death - Lauren F Friedman (Business Insider) | http://www.businessinsider.com.au/philip-seymour-hoffman-overdose-2014-2
- ↑ Siegel, S., Hinson, R. E., Krank, M. D., McCully, J. (23 April 1982). "Heroin "Overdose" Death: Contribution of Drug-Associated Environmental Cues". Science. 216 (4544): 436–437. doi:10.1126/science.7200260. ISSN 0036-8075.
- ↑ Winger, G., Hursh, S. R., Casey, K. L., Woods, J. H. (1 May 2002). "Relative Reinforcing Strength of ThreeN-Methyl-d-Aspartate Antagonists with Different Onsets of Action". Journal of Pharmacology and Experimental Therapeutics. 301 (2): 690–697. doi:10.1124/jpet.301.2.690. ISSN 0022-3565.
- ↑ 24.0 24.1 Ershad, M., Cruz, M. D., Mostafa, A., Mckeever, R., Vearrier, D., Greenberg, M. I. (March 2020). "Opioid Toxidrome Following Grapefruit Juice Consumption in the Setting of Methadone Maintenance". Journal of Addiction Medicine. 14 (2): 172–174. doi:10.1097/ADM.0000000000000535. ISSN 1932-0620.
- ↑ https://www.health.act.gov.au/about-our-health-system/population-health/drug-law-reform
- ↑ Vancouver Insite drug-injection facility can stay open, 2011
- ↑ http://scc.lexum.org/en/2011/2011scc44/2011scc44.html
- ↑ New drug guidelines are Europe’s most liberal
- ↑ {Citation | title=Anlage I BtMG - Einzelnorm | url=http://www.gesetze-im-internet.de/btmg_1981/anlage_i.html}}
- ↑ Anlage II BtMG - Einzelnorm
- ↑ Anlage III BtMG - Einzelnorm
- ↑ [1] Tabella 1 delle sostanze stupefacenti e psicotrope, page 12. (text in italian).
- ↑ Zaudējis spēku - Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem
- ↑ Постановление Правительства РФ от 01.10.2012 N 1002 (ред. от 09.08.2019)
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.

