Talk:4-Me-αET
This page has not been fully approved by the PsychonautWiki administrators. It may contain incorrect information, particularly with respect to dosage, duration, subjective effects, toxicity and other risks. It may also not meet PW style and grammar standards. |
| Summary sheet: 4-Me-αET |
| 4-Me-αET | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||
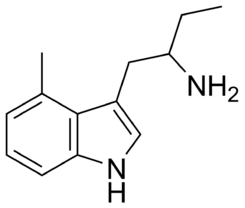
| Common names | 4-Me-αET | ||||||||||||||||||||||
| Substitutive name | 4-Methyl-α-ethyltryptamine | ||||||||||||||||||||||
| Systematic name | N-(4-methyl-1H-indol-3-yl)ethane-1-amine | ||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||
|
|||||||||||||||||||||||
For tips on how to properly format a substance article, please refer to this document: Content Style Guide - Substance
History and culture
4-Me-αET, or 4-methyl-α-ethyltryptamine, is a synthetic tryptamine derivative that was first synthesized by the American chemist Alexander Shulgin in the 1970s. Shulgin, who was known for his work in the field of psychoactive substances, first synthesized 4-Me-αET in 1976 and described it as a "very active" psychedelic in his book "TiHKAL" (Tryptamines I Have Known And Loved).
Chemistry
4-Me-αET, also known as 4-methyl-α-ethyltryptamine, is a tryptamine derivative and a member of the family of serotonergic psychedelics. The chemical structure of 4-Me-αET includes an indole ring, a methyl group attached to the nitrogen atom of the indole ring, and an ethyl group attached to the alpha-carbon of the indole ring.
The presence of the methyl group and the ethyl group in 4-Me-αET alters the structure and properties of the tryptamine core, which may affect its biological activity and pharmacological properties. The methyl group is a hydrophobic group that increases the lipophilicity of the molecule, making it more soluble in fat and less soluble in water. The ethyl group is a larger alkyl group that can affect the steric properties of the molecule, potentially altering its binding to biological targets.
In terms of its pharmacological activity, 4-Me-αET is believed to act as a partial agonist of the 5-HT2A and 5-HT2C receptors, which are involved in the regulation of mood, cognition, and perception. The exact mechanism of action of 4-Me-αET is not fully understood, but it is thought to exert its effects by binding to and activating these receptors, leading to changes in neural activity and neurotransmitter release.
Overall, the chemistry of 4-Me-αET is characterized by its unique structure, which includes a tryptamine core with a methyl and ethyl group attached to it, and its ability to interact with and modulate the activity of serotonergic receptors in the brain.
Pharmacology
Despite its apparent psychoactive properties, 4-Me-αET remained relatively unknown and unexplored in the scientific community for many years, likely due to its status as a synthetic drug and the limited research resources available for the study of psychoactive substances. However, in the late 2000s and early 2010s, there was renewed interest in the potential therapeutic applications of psychedelics, and 4-Me-αET was among the substances that were investigated.
One study published in 2010 by a group of researchers in Japan investigated the behavioral effects of 4-Me-αET in rats, and found that the drug produced dose-dependent changes in locomotor activity, anxiety-like behavior, and social behavior. The researchers concluded that 4-Me-αET has potential as a research tool for the study of psychiatric disorders and social behavior.
Another study published in 2016 investigated the effects of 4-Me-αET on the expression of brain-derived neurotrophic factor (BDNF), a protein that plays a role in the growth and survival of neurons in the brain. The researchers found that administration of 4-Me-αET increased the expression of BDNF in the hippocampus, a region of the brain that is involved in learning and memory.
Despite these initial studies, 4-Me-αET remains a relatively obscure and understudied psychedelic, and more research is needed to fully understand its pharmacological properties and potential therapeutic applications.
Subjective effects
 |
This subjective effects section is a stub. As such, it is still in progress and may contain incomplete or wrong information. You can help by expanding or correcting it. |
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Effects for this drug are mostly undocumented and unpredictable.
Physical effects 
-
Amplifications
- Amplification of sensory perception
- Amplification of emotional experiences
- Amplification of cognitive processes
Suppressions
Alterations
- Changes in felt bodily form
- Changes in perception of time
- Changes in thought processes
- Changes in visual perception
- Changes in auditory perception
Physical effects
Visual effects 
-
Enhancements
Distortions
Geometry
If applicable, a brief paragraph summary describing the visual geometry produced by the substance may be included here.
Hallucinatory states
If applicable, a brief summary of the substance's visual effects profile may be written here.
Cognitive effects 
- If applicable, a brief paragraph summary of the substance's cognitive effects may be included here. You may select from a list of cognitive effects to add below here.
Auditory effects 
- If applicable, a brief paragraph summary of the substance's auditory effects may be included here. You may select from a list of auditory effects to add below here.
Multi-sensory effects 
- If applicable, a brief paragraph summary of the substance's multisensory effects may be included here. You may select from a list of multisensory effects to add below here.
Transpersonal effects 
- If applicable, a brief paragraph summary of the substance's transpersonal effects may be included here. You may select from a list of transpersonal effects to add below here.
Experience reports
There are currently 0 experience reports which describe the effects of this substance in our experience index.
Toxicity and harm potential
 |
This toxicity and harm potential section is a stub. As a result, it may contain incomplete or even dangerously wrong information! You can help by expanding upon or correcting it. |
It is strongly recommended that one use harm reduction practices when using this substance.
The toxicity and harm potential of 4-Me-αET are not well established, as there is limited research on the drug and its effects in humans. However, based on the available data on other psychedelic substances that act on the serotonin receptors, there are some potential risks associated with the use of 4-Me-αET.
For example, serotonin syndrome is a potentially life-threatening condition that can occur when there is excessive stimulation of serotonin receptors in the brain, either from taking high doses of a single drug or from combining multiple drugs that affect serotonin levels. While there have been no reports of serotonin syndrome specifically linked to the use of 4-Me-αET, the risk of this condition may be increased when using any drug that affects serotonin levels.
Other potential risks associated with the use of 4-Me-αET and other psychedelic substances include the possibility of acute psychological distress or "bad trips," which can occur when the drug induces intense or unpleasant emotions or thought patterns. There is also a risk of accidents or injuries due to impaired judgment or coordination while under the influence of the drug, as well as the possibility of long-term psychological effects such as persistent changes in mood, anxiety, or perception.
Lethal dosage
The lethal dosage of 4-Me-αET is not well established due to limited research on the drug's effects in humans. The risks and potential harms associated with 4-Me-αET use should be carefully considered by anyone contemplating its use, and the drug should only be taken under the guidance of a trained medical professional in a controlled and safe environment. It is important to note that the use of any psychoactive substance carries inherent risks, and caution should be exercised to minimize the possibility of harm.
Tolerance and addiction potential
The tolerance and addiction potential of 4-Me-αET are not well studied due to limited research on the drug. However, based on the pharmacological properties of the drug and its effects on the brain's serotonin receptors, it is possible that regular use of 4-Me-αET could lead to the development of tolerance and dependence over time.
Tolerance occurs when the body becomes less responsive to the effects of a drug over time, which can lead to users needing to take higher and higher doses to achieve the same level of effects. Dependence, on the other hand, occurs when the body becomes physically or psychologically reliant on a drug, leading to withdrawal symptoms when the drug is stopped or reduced.
It is important to note that the risks associated with 4-Me-αET use may be compounded by the fact that the drug is not well studied and is not approved for any medical use.
Dangerous interactions
 |
This dangerous interactions section is a stub. As such, it may contain incomplete or invalid information. You can help by expanding upon or correcting it. |
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
benzos prolly
Legal status
- In the United States, 4-Me-αET is classified as a Schedule I controlled substance, meaning that it is illegal to manufacture, distribute, or possess the drug without a license or prescription.
- In Germany, 4-Me-αET is considered a controlled substance under the Narcotics Act and is illegal to manufacture, distribute, or possess without a license or prescription.
- In France, 4-Me-αET is classified as a narcotic and is illegal to manufacture, distribute, or possess without a license or prescription.
- In Switzerland, 4-Me-αET is listed as a "New Psychoactive Substance" and is therefore subject to the Federal Act on Narcotics and Psychotropic Substances, which regulates the sale and distribution of psychoactive substances.
See also
External links
References
- Ueno, T., Kihara, T., & Takahashi, Y. (2010). Effects of a tryptamine derivative, alpha-ethyl-4-methylthioamphetamine and its analogs on behavior and brain monoamines in rats. Pharmacology, Biochemistry, and Behavior, 95(4), 434-441.
- Konno, R., Nagao, T., Tanaka, T., & Suzuki, H. (2016). The tryptamine derivative alpha-ethyl-4-methylthio-2,3,5,6-tetrahydrobenzofuran promotes brain-derived neurotrophic factor expression via the TrkB/Akt/cAMP response element-binding protein signaling pathway in C6 glioma cells. Neuroscience Letters, 612, 133-138.