Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical isomers, and others. There are two main forms of isomerism: structural isomerism and stereoisomerism (spatial isomerism).
Forms of Isomerism
Structural isomers
In structural isomers, sometimes referred to as constitutional isomers, the atoms, and functional groups are joined in different ways. The isomers have the same amount of each molecule, but the molecules are arranged in different ways. Structural isomers have different IUPAC names and may or may not belong to the same functional group. This group includes chain isomerism whereby hydrocarbon chains have variable amounts of branching. Position isomerism deals with the position of a functional group on a chain, and functional group isomerism is when one functional group is split up into different ones.
Stereoisomers
In stereoisomers, the bond structure is the same, but the geometrical positioning of atoms and functional groups in space differs. This class includes enantiomers which are non-superimposable mirror images of each other, and diastereomers which are not. Enantiomers always contain chiral centers, and diastereomers often do, but there are some diastereomers which are neither chiral nor contain chiral centers. Another type of isomer, conformational isomers (conformers), may be rotamers, diastereomers or enantiomers depending on the specific compound.
Diastereomers
Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent stereocenters and are not mirror images (enantiomers) of each other. When two diastereoisomers differ from each other at only one stereocenter, they are epimers. Each stereocenter gives rise to two different configurations and thus increases the number of stereoisomers by a factor of two.
"Cis–trans isomers" are used to describe any molecules with restricted rotation in the molecule. However, these descriptors only describe relative stereochemistry based on group bulkiness or principal carbon chain so that it can be ambiguous. This is especially problematic for double bonds that have more than two substituents.
E/Z isomers which have restricted rotation within the molecule, specifically isomers containing a double bond, are configurational isomers. For alkenes with more than two substituents, the E-Z notation is used instead of cis and trans. If possible, E and Z is also preferred in compounds with two substituents.
Enantiomers
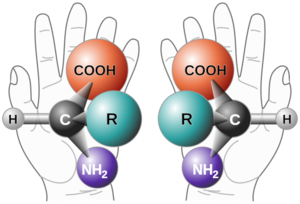
An enantiomer is one of two stereoisomers that are mirror images of each other that are non-superimposable (not identical). The model used to convey the idea of isomers being non-superimposable is a person's hands. One's left and right hands are the same except for opposite orientation. Organic compounds that contain a chiral carbon usually have two non-superimposable structures. A chiral carbon is a carbon attached to four completely different functional groups. The enantiomers will have a "left-hand" and a "right-hand" molecule. This property is used in naming the isomers: the "right-hand" is given the prefix (R), and the "left-hand" is given the prefix (S). Enantiomers rotate in plane-polarized light, and this property is also used in naming the enantiomers as either clockwise (prefix of dextro- or +) or anti-clockwise (prefix of levo or -).
Nomenclature
Often, the D/L system (with small caps) and the d/l system (with lower-case) are confused. The result is that the levorotary l-ephedrine is wrongly named L-ephedrine and the dextrorotary d-pseudoephedrine (the diastereomer) wrongly D-pseudoephedrine.
Chirality
A chiral molecule is a type of molecule that has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom, where the carbon is attached to four entirely different functional groups.
Most biological molecules (proteins, sugars, et al.) are present in only one of many chiral forms, so different enantiomers of a chiral drug molecule bind differently (or not at all) to target receptors. One enantiomer of a drug may have a desired beneficial effect while the other may cause severe and undesired side effects, or sometimes even beneficial but entirely different effects.
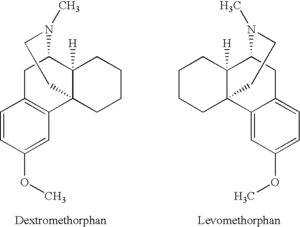
One example of this is with the dissociative drug DXM. DXM, or dextromethorphan, is the isomer that rotates clockwise in plane-polarized light. It has antitussive (cough suppressant) and dissociative effects. Levomethorphan is the isomer that rotates anti-clockwise in plane-polarized light. This difference in rotation can radically alter the effects and character of the drug; for example, levomethorphan is an opioid painkiller.
Racemic mixture
In chemistry, a racemic mixture, or racemate, is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. A racemic mixture is the most common forms chemicals (psychoactive or not) end up synthesized as.
A racemate is optically inactive, meaning that there is no net rotation of plane-polarized light. Although the two enantiomers rotate plane-polarized light in opposite directions, the rotations cancel because they are present in equal amounts. In contrast to the two pure enantiomers, which have identical physical properties except for the direction of rotation of plane-polarized light, a racemate sometimes has different properties from either of the pure enantiomers. Different melting points are most common, but different solubilities and boiling points are also possible.[1]
Pharmaceuticals may be available as a racemate or as the pure enantiomer (enantiopure), which might have different potencies and effects. Because biological systems have many chiral asymmetries, pure enantiomers frequently have very different biological effects. Examples include glucose and methamphetamine.[2]
Enantiopure
A sample with only a single enantiomer is an enantiomerically pure, enantiopure or homochiral compound.
See also
External links
References
- ↑ https://en.wikipedia.org/wiki/Racemic_mixture | Racemic mixture (Wikipedia)
- ↑ https://en.wikipedia.org/wiki/Racemic_mixture | Racemic mixture (Wikipedia)