Talk:Morphine/Archive 1
This page has been moved to an archived sub page in order to make room for the Flow extension.
This article is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
Morphine is a naturally occurring opiate alkaloid found within the latex of seed pods produced by the Opium Poppy (Papaver Somniferum) and in lower concentrations within other species of Papaver.
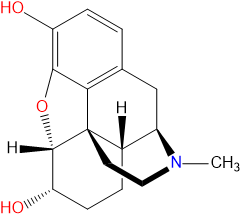
Chemistry
Pharmacology
Its duration of analgesia is about 3–4 hours when administered via the intravenous, subcutaneous, or intramuscular route and 3–6 hours when given by mouth. Morphine is also used in slow release formulations for opiate substitution therapy (OST) in Austria, Bulgaria, and Slovenia, for addicts who cannot tolerate the side effects of using either methadone or buprenorphine, or for addicts who are "not held" by buprenorphine or methadone. It is used for OST in many parts of Europe although on a limited basis.
Endogenous opioids include endorphins, enkephalins, dynorphins, and even morphine itself. Morphine appears to mimic endorphins. Endorphins, a contraction of the term endogenous morphines, are responsible for analgesia (reducing pain), causing sleepiness, and feelings of pleasure. They can be released in response to pain, strenuous exercise, orgasm, or excitement.
Morphine is the prototype narcotic drug and is the standard against which all other opioids are tested. It interacts predominantly with the μ-opioid receptor. These μ-binding sites are discretely distributed in the human brain, with high densities in the posterior amygdala, hypothalamus, thalamus, nucleus caudatus, putamen, and certain cortical areas. They are also found on the terminal axons of primary afferents within laminae I and II (substantia gelatinosa) of the spinal cord and in the spinal nucleus of the trigeminal nerve.
Morphine is a phenanthrene opioid receptor agonist – its main effect is binding to and activating the μ-opioid receptors in the central nervous system. In clinical settings, morphine exerts its principal pharmacological effect on the central nervous system and gastrointestinal tract. Its primary actions of therapeutic value are analgesia and sedation. Activation of the μ-opioid receptors is associated with analgesia, sedation, euphoria, physical dependence, and respiratory depression. Morphine is a rapid-acting narcotic, and it is known to bind very strongly to the μ-opioid receptors, and for this reason, it often has a higher incidence of euphoria/dysphoria, respiratory depression, sedation, pruritus, tolerance, and physical and psychological dependence when compared to other opioids at equianalgesic doses Morphine is also a κ-opioid and δ-opioid receptor agonist, κ-opioid's action is associated with spinal analgesia, miosis (pinpoint pupils) and psychotomimetic effects. δ-opioid is thought to play a role in analgesia. Although morphine does not bind to the σ-receptor, it has been shown that σ-agonists, such as (+)-pentazocine, antagonize morphine analgesia, and σ-antagonists enhance morphine analgesia, suggesting some interaction between morphine and the σ-opioid receptor.
The effects of morphine can be countered with opioid antagonists such as naloxone and naltrexone; the development of tolerance to morphine may be inhibited by NMDA antagonists such as ketamine or dextromethorphan. The rotation of morphine with chemically dissimilar opioids in the long-term treatment of pain will slow down the growth of tolerance in the longer run, particularly agents known to have significantly incomplete cross-tolerance with morphine such as levorphanol, ketobemidone, piritramide, and methadone and its derivatives; all of these drugs also have NMDA antagonist properties. It is believed that the strong opioid with the most incomplete cross-tolerance with morphine is either methadone or dextromoramide.
Morphine can be taken orally, sublingually, bucally, rectally, subcutaneously, intravenously, intrathecally or epidurally and inhaled via a nebulizer. On the streets, it is becoming more common to inhale (“Chasing the Dragon"), but, for medical purposes, intravenous (IV) injection is the most common method of administration. Morphine is subject to extensive first-pass metabolism (a large proportion is broken down in the liver), so, if taken orally, only 40–50% of the dose reaches the central nervous system. Resultant plasma levels after subcutaneous (SC), intramuscular (IM), and IV injection are all comparable. After IM or SC injections, morphine plasma levels peak in approximately 20 minutes, and, after oral administration, levels peak in approximately 30 minutes.[39] Morphine is metabolised primarily in the liver and approximately 87% of a dose of morphine is excreted in the urine within 72 hours of administration. Morphine is metabolized primarily into morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G) via glucuronidation by phase II metabolism enzyme UDP-glucuronosyl transferase-2B7 (UGT2B7). About 60% of morphine is converted to M3G, and 6–10% is converted to M6G.[41] Not only does the metabolism occur in the liver but it may also take place in the brain and the kidneys. M3G does not undergo opioid receptor binding and has no analgesic effect. M6G binds to μ-receptors and is half as potent an analgesic as morphine in humans. Morphine may also be metabolized into small amounts of normorphine, codeine, and hydromorphone. Metabolism rate is determined by gender, age, diet, genetic makeup, disease state (if any), and use of other medications. The elimination half-life of morphine is approximately 120 minutes, though there may be slight differences between men and women. Morphine can be stored in fat, and, thus, can be detectable even after death. Morphine is able to cross the blood–brain barrier, but, because of poor lipid solubility, protein binding, rapid conjugation with glucuronic acid and ionization, it does not cross easily. Diacetylmorphine, which is derived from morphine, crosses the blood–brain barrier more easily, making it more potent.
There are also slow-release formulations of orally administered morphine whose effect last substantially longer than bare morphine, availing for one administration per day.
Subjective effects
Toxicity and harm potential
A large overdose can cause asphyxia and death by respiratory depression if the person does not receive medical attention immediately. Overdose treatment includes the administration of naloxone. The latter completely reverses morphine's effects, but precipitates immediate onset of withdrawal in opiate-addicted subjects. Multiple doses may be needed.
The minimum lethal dose is 200 mg but in case of hypersensitivity 60 mg can bring sudden death. In serious drug dependency (high tolerance), 2000-3000 mg per day can be tolerated.