MDMA
| Summary sheet: MDMA |
| MDMA | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||
| Common names | MDMA, Molly, Mandy, Emma, MD, Ecstasy, E, X, XTC, Rolls, Beans, Pingers | ||||||||||||||||||||||||||||||||
| Substitutive name | 3,4-Methylenedioxy-N-methylamphetamine | ||||||||||||||||||||||||||||||||
| Systematic name | (RS)-1-(Benzo[d][1,3]dioxol-5-yl)-N-methylpropan-2-amine | ||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||
| Psychoactive class | Stimulant / Entactogen | ||||||||||||||||||||||||||||||||
| Chemical class | Amphetamine / MDxx | ||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||
| 5-MeO-xxT | |||||||||||||||||||||||||||||||||
| Alcohol | |||||||||||||||||||||||||||||||||
| Cocaine | |||||||||||||||||||||||||||||||||
| DOx | |||||||||||||||||||||||||||||||||
| GHB | |||||||||||||||||||||||||||||||||
| GBL | |||||||||||||||||||||||||||||||||
| MXE | |||||||||||||||||||||||||||||||||
| ΑMT | |||||||||||||||||||||||||||||||||
| Protease Inhibitors | |||||||||||||||||||||||||||||||||
| SSRIs | |||||||||||||||||||||||||||||||||
| SNRIs | |||||||||||||||||||||||||||||||||
| 25x-NBOMe | |||||||||||||||||||||||||||||||||
| PCP | |||||||||||||||||||||||||||||||||
| Serotonin releasers | |||||||||||||||||||||||||||||||||
| 2C-T-x | |||||||||||||||||||||||||||||||||
| 5-HTP | |||||||||||||||||||||||||||||||||
| Tramadol | |||||||||||||||||||||||||||||||||
| MAOIs | |||||||||||||||||||||||||||||||||
| DXM | |||||||||||||||||||||||||||||||||
3,4-Methylenedioxymethamphetamine (also known as ecstasy, E, XTC, emma, molly, mandy, pingers, and MDMA) is a classical entactogen substance of the amphetamine class. It is the most well-known and widely-used member of the entactogens, a diverse group that includes MDA, methylone, 4-MMC, and 6-APB. It produces its effects by promoting the release of serotonin, dopamine, and norepinephrine in the brain.
MDMA was first developed in 1912 by the pharmaceutical company Merck.[1] However, human use was not reported until the 1970s, when it became known to underground psychotherapy circles in the United States.[2] In the early 1980s, it spread into nightlife and rave culture, which eventually resulted in federal scheduling in 1985.[3] By 2014, it was estimated to be one of the most popular recreational drugs in the world, alongside cocaine and cannabis.[4]
Today, recreational MDMA use is widely associated with dance parties, electronic dance music, and the clubbing and raving scenes.[5] Researchers are currently investigating whether MDMA may assist in treatment-resistant post-traumatic stress disorder (PTSD), social anxiety in autistic adults,[6] and anxiety in those with life-threatening illness.[7][8][9]
Subjective effects include stimulation, anxiety suppression, disinhibition, enhanced empathy and sociability, relaxation, and euphoria. It is classified as an entactogen due to how it facilitates feelings of closeness with one's self and others. A notable property of MDMA is that tolerance builds unusually quickly and many users report that it dramatically loses effectiveness if used on a frequent basis.
It is usually recommended to wait for one to three months between each use to give the brain adequate time to restore serotonin levels and avoid toxicity. Additionally, using excessively high doses and multiple redosing is highly discouraged as this is thought to significantly increase the toxicity of MDMA.
MDMA has moderate to high abuse potential and can produce psychological dependence in some users. Acute adverse effects are usually the result of high or multiple doses, although single dose toxicity can occur in susceptible individuals.[10] The most serious short-term physical health risks of MDMA are overheating and dehydration, which has resulted in deaths.[11] It has also been shown to be neurotoxic at high doses;[12] however, it is unclear how much this risk applies to typical recreational usage.[13] MDMA may cause excessive thirst, and inability to urinate the water, which may cause water intoxication and electrolyte imbalance. MDMA has been shown to cause sexual dysfunction, including erectile dysfunction and delayed orgasm (see subjective effects).
It is highly advised to use harm reduction practices if using this substance.
History and culture
MDMA was first synthesized in 1912 by the German chemist Dr. Anton Köllisch while employed at the pharmaceutical company Merck. Köllisch was in the process of developing agents that would help manage excess bleeding and was interested in MDMA synthesis because it was an intermediate in the production of methylhydrastinin, the methylated analog of the hemostatic agent hydrastinine. There are no indications of interest in MDMA as an active agent itself.[1]
It was not mentioned again until 1927, when Dr. Max Oberlin conducted the first proven pharmacological tests at Merck while searching for compounds with a similar action spectrum to adrenaline or ephetonine. Despite promising results, research was halted due to rising substance prices.[1]
In 1965, the American chemist Alexander Shulgin synthesized MDMA as an academic exercise but did not test it for psychoactivity.[14][2] Shulgin claims to have first heard about the effects of MDMA in 1967 from a student and decided to experiment with it himself. He was impressed with the effects of the substance and believed it could have therapeutic utility. He advertised it to therapists and psychiatrists which led it to gain some popularity as an adjunct treatment for various psychological disorders.[2]
During this period, psychotherapist Dr. Leo Zeff came out of retirement and subsequently introduced the then-legal MDMA to over 4,000 patients. From the mid-1970s to the mid-1980s there was a growth of clinicians using MDMA (then known as "Adam") in California.[15]
Recreational use of MDMA became popular at around the same time, particularly in nightclubs, eventually catching the attention of the Drug Enforcement Administration (DEA). After several hearings, a US Federal Administrative Law Judge recommended that MDMA should be made a Schedule III controlled substance so that it could be used in the medical field. Despite this, the director of the DEA overruled this recommendation and classified MDMA as a Schedule I controlled substance.[16][14]
In the United Kingdom, the 1971 Misuse of Drugs Act, which had already been altered in 1977 to include all ring-substituted amphetamines like MDMA, was further amended in 1985 to refer specifically to Ecstasy, placing it in the Class A category.[15]
Chemistry
MDMA, or 3,4-methylenedioxy-N-methylamphetamine, is a synthetic molecule of the substituted amphetamine class. Molecules of the amphetamine class all contain a phenethylamine core comprised of a phenyl ring bound to an amino (NH2) group through an ethyl chain, with an additional methyl substitution at Rα. In addition to this, MDMA contains a methyl substitution on RN, a feature it shares with methamphetamine. Critically, the MDMA molecule also contains substitutions at R3 and R4 of the phenyl ring with oxygen groups -- these oxygen groups are incorporated into a methylenedioxy ring through a methylene bridge. MDMA shares this methylenedioxy ring with other entactogens and stimulants like MDA, MDEA and MDAI.
Pharmacology
MDMA acts primarily as a releasing agent of the three principal monoamine neurotransmitters serotonin, norepinephrine, and dopamine through its action at trace amine-associated receptor 1 (TAAR1) and vesicular monoamine transporter 2 (VMAT2).[17][18][19] MDMA is a monoamine transporter substrate (i.e. a substrate for the transporters for dopamine (DAT), norepinephrine (NET), and serotonin (SERT)), enabling it to enter monoaminergic neurons via these neuronal membrane transport proteins.[18] By acting as a monoamine transporter substrate, MDMA produces competitive reuptake inhibition at the neuronal membrane transporters, competing for endogenous monoamines for reuptake.[18][20]
MDMA inhibits both vesicular monoamine transporters (VMATs), the second of which (VMAT2) is highly expressed within monoamine neurons vesicular membranes.[19] Once inside a monoamine neuron, MDMA acts as a VMAT2 inhibitor and a TAAR1 agonist.[18][21] The inhibition of VMAT2 by MDMA results in increased concentrations of the aforementioned monoamine neurotransmitters in the cytosol of the neuron.[19][22] Activation of TAAR1 by MDMA triggers protein kinase signaling events which then phosphorylates the associated monoamine transporters of the neuron.[18]
Subsequently, these phosphorylated monoamine transporters either reverse transport direction – i.e. move neurotransmitters from inside the cell to the synaptic cleft – or withdraw into the neuron, respectively producing the inflow of neurotransmitters and noncompetitive reuptake inhibition at the neuronal membrane transporters.[18] MDMA has ten times more affinity for uptake at serotonin transporters compared to dopamine and norepinephrine transporters and consequently has mainly serotonergic effects.[23]
MDMA also has weak agonist activity at postsynaptic serotonin receptors 5-HT1 and 5-HT2 receptors, and its more efficacious metabolite MDA likely augments this action.[24][25][26][27] Cortisol, prolactin, and oxytocin quantities in serum are increased by MDMA.[28]
Additionally, MDMA is a ligand at both sigma receptor subtypes, though its efficacies at these receptors and the role that they play have yet to be elucidated.[29]
Subjective effects
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Abnormal heartbeat[citation needed]
- Appetite suppression
- Bodily control enhancement
- Bronchodilation[citation needed]
- Dehydration -- Avoid drinking too much water, see the water intoxication and electrolyte imbalance section.
- Difficulty urinating - Avoid drinking too much water, see the water intoxication and electrolyte imbalance section.
- Dry mouth - Avoid drinking too much water, see the water intoxication and electrolyte imbalance section.
- Excessive yawning - Excessive yawning is thought to occur as a result of serotonergic activity (similar to psilocybin mushrooms) and is more likely to occur with higher doses or pure MDMA. It is sometimes used as an indicator of a batch's quality.
- Increased blood pressure[30][31]
- Increased bodily temperature[32] - As MDMA is a serotonin releasing agent, a rise in core body temperature tends to be a significant and consistent part of the experience. Caution must be taken as too high of a dose in a dangerously hot environment can result in serotonin toxicity, which can be fatal if left untreated. Avoid drinking too much water, see the water intoxication and electrolyte imbalance section.
- Increased heart rate[30]
- Increased perspiration[33] - Avoid drinking too much water, see the water intoxication and electrolyte imbalance section.
- Muscle contractions
- Nausea - This effect is most commonly present during the come up phase of the experience, and at higher doses, but has been reported to occur spontaneously in those who seem to be susceptible to it.
- Neurotoxicity - Long-term use can lead to a significant degree of neurotoxicity.
- Pain relief - This effect is generally not as powerful as it is with opioids.[34][35][36]
- Pupil dilation
- Seizure - Seizures are rare but may occur in those who are susceptible to them, especially when taking higher doses or redosing while in physically taxing conditions such as being dehydrated, fatigued, undernourished, or overheated.[citation needed]
- Temporary erectile dysfunction & Orgasm suppression[37][38]
- Spontaneous bodily sensations - The "body high" of MDMA can be characterized as a moderate to extreme euphoric tingling sensation that encompasses the entire body. This sensation maintains a consistent presence that steadily rises with the onset and hits its limit once the peak has been reached.
- Physical euphoria - Physical euphoria is a prominent aspect of the MDMA experience and occurs reliably when MDMA is used responsibly (i.e. reasonable dosing and spacing between experiences) and can lead to profound feelings of social and physical disinhibition. However, euphoria is quick to fade as one builds tolerance to MDMA's effects, colloquially known as "losing the magic".
- Stamina enhancement
- Stimulation - MDMA is popularly known for being stimulating and energetic. This encourages activities such as running, climbing and dancing in a way that makes MDMA a popular choice for musical events such as festivals and raves. The distinct style of stimulation which MDMA presents can be described as forced. This means that at higher doses, it becomes difficult or impossible to keep still as jaw clenching, involuntarily body shakes and vibrations become present, resulting in an unsteadiness of the hands and a general lack of motor control. Unlike most other stimulants, however, the stimulating effects of MDMA can also paradoxically be accompanied by persistent or wave-like feelings of deep sedation and relaxation, typically at moderate to strong doses. This may be also be accompanied with a more encourged style of stimulation for some individuals as a result, where one can engage in stimulating acitvities like dancing if they so choose, but can also relax and unwind without feeling restless if they so choose.
- Tactile enhancement - MDMA produces distinct enhancements to tactile sensations. Users commonly report a sense of softness and fuzziness draping over their skin. Likewise, touching soft and fuzzy objects such as shag rugs can become irresistibly pleasurable and satisfying. MDMA-type tactile enhancement appears to be an effect unique to the entactogen class and may be a serotonin-related effect.
- Teeth grinding[39] - This effect when experienced alongside the cognitive and physical euphoria can often lead to users mildly or intensely clenching their jaw muscles, sometimes even to the point where the individual’s facial expression begins to change. This is sometimes colloquially called “gurning”[40] and is typically only experienced in moderate to high dosages. For other individuals, especially if they are prone to experience the paradoxical feelings of muscle relaxation, bruxism may not be as pronounced.
- Temperature regulation suppression - Avoid drinking too much water, see the water intoxication and electrolyte imbalance section.
- Vasoconstriction
- Vibrating vision - At high doses, a person's eyeballs may begin to spontaneously wiggle back and forth in a rapid motion, causing the vision to become blurry and temporarily out of focus. This is a condition known as nystagmus.
Visual effects 
-
The visual effects of MDMA occur more selectively and less consistently than any of the traditional psychedelics. This has resulted in many people disregarding the psychedelic aspects of MDMA as a myth or rumor, despite a large body of anecdotal reports suggesting otherwise. The effects can not be guaranteed to manifest themselves, but are the most likely to occur with chemically pure MDMA at high doses, towards the end of the experience and particularly if the user has been smoking cannabis. They also seem more likely to occur if the user has prior experience with psychedelics.
Enhancements
MDMA presents an array of visual enhancements which are mild in comparison to traditional psychedelics, but still distinctly present. These generally include:
Suppressions
Distortions
Geometry
The visual geometry produced by MDMA can be characterized as more similar in appearance to that of psilocin than LSD. It can be comprehensively described through its variations as primarily intricate in complexity, abstract in form, organic in style, structured in organization, dimly lit in lighting, mostly monotone in colour with blues and greys, glossy in shading, sharp in edges, small in size, fast in speed, smooth in motion, equal in round and angular corners, non-immersive in-depth and consistent in intensity. At higher doses, they are significantly more likely to give rise to states of level 8A visual geometry over level 8B. Many users report that MDMA geometry presents itself with dark and menacing emotional vibes with a synthetic and nerve-racking feel to them.
Hallucinatory states
MDMA is capable of producing a unique range of low and high-level hallucinatory states in a fashion that is significantly less consistent and reproducible than that of most other commonly used psychedelics. These effects are far more common during either the very peak or offset of the experience and commonly include:
- External hallucination (autonomous entities; settings, sceneries, and landscapes; perspective hallucinations and scenarios and plots) - This effect shares many similarities to those produced by deliriant substances, but does not manifest itself consistently and usually happens only at heavy, likely toxic doses. It can be comprehensively described through its variations as delirious in believability, autonomous in controllability and solid in style. They usually follow themes of memory replays and semi-realistic or expected events. For example, people could be casually holding objects or performing actions which one would expect them to be in real life before disappearing and dissolving under further inspection. Common examples of this include seeing people wearing glasses, or hats when they are not and mistaking objects for human beings or animals.
- Internal hallucinations - The internal hallucinations which MDMA induces are only present as spontaneous breakthroughs at extremely high doses.[citation needed] This effect's variations are delirious in believability, interactive in style, new experiences in content, autonomous in controllability and solid in appearance. The most common way in which they manifest themselves is through hypnagogic scenarios which the user may experience as they are drifting off to sleep after a night of use; these can usually be described as memory replay from the previous several hours. These are brief and fleeting, but frequent and completely believable and convincing as they happen. In terms of the theme, they often are in the form of conversations with people or instead manifest as bizarre and extremely nonsensical plots.
- Peripheral information misinterpretation[citation needed]
Cognitive effects 
-
The general head space of MDMA is described by many as one of pronounced mental stimulation, feelings of love, empathy, openness and a pronounced sense of rejuvenation and euphoria. It is capable of producing a large number of cognitive effects that are typically associated with entactogens and stimulants.
The most prominent of these effects include:
- Amnesia - Very high doses of MDMA can sometimes cause partial amnesia.
- Anxiety suppression
- Cognitive euphoria - Strong emotional euphoria and feelings of happiness are present in MDMA and are likely a direct result of a concerted mechanism of serotonin, norepinephrine, and dopamine release.[citation needed]
- Compulsive redosing
- Creativity enhancement
- Decreased libido[41][42][43]
- Delirium & Confusion - This effect typically only occurs with overly high doses, and is associated with temperature dysregulation and overheating, particularly when MDMA is taken in crowded, physically strenuous environments that leaves the user unable to cool off, rest, or rehydrate adequately.
- Disinhibition
- Ego inflation
- Emotion enhancement
- Empathy, affection, and sociability enhancement - This particular effect is usually more consistent, pronounced, powerful and therapeutic with MDMA than any other known substance. It is the most evident and noticeable effect within any MDMA experience and dominates the head space. With time and repeated use, however, this effect becomes severely diminished as the perspective it instills becomes fully grounded and already in place, making people feel merely stimulated and euphoric with no new found urges to communicate with others. Some users report that MDMA "loses its magic" with as few as ten experiences, while others have reported hundreds of uses before the empathic qualities disappear. This does not appear to be valid for all users, however, with many users reporting that they have not experienced any decrease in quality of the experience despite dozens or even hundreds of uses.
- Focus enhancement - Focus enhancement only occurs at low to moderate doses. Higher doses will usually impair attention and concentration, particularly during the "come down" phase of the experience.
- Immersion enhancement
- Increased music appreciation -This effect usually manifests itself through an increase in comprehension of subtle details in the music, a feeling of deeper understanding with regards to composition and lyrics and an increased enthusiasm towards hearing music in general. Rarely, one may find music to be annoying or distracting rather than enhanced - this seems to be largely set and setting based and happens rarely.
- Increased sense of humor
- Mindfulness
- Motivation enhancement
- Rejuvenation
- Thought acceleration
- Time compression - Strong feelings of time compression are commonly produced by MDMA and speed up the experience of time noticeably.
- Wakefulness
Auditory effects 
-
- Enhancements
- Hallucinations
- Distortions
- Tinnitus - Tinnitus is rarely reported, but typically manifests as a muffled roaring in the ears, affected by whether the user is upright or laying down. It is most commonly reported when using in conjunction with other substances but can manifest on its own at higher doses. This may be accompanied by partial or total, yet highly temporary (on the order of a minute), hearing loss, especially when standing. Some users have reported acquiring permanent tinnitus after abuse.
Transpersonal effects 
-
- Existential self-realization - Although present, this effect is not quite as pronounced or as consistent when compared to hallucinogens such as mescaline, psilocybin, LSD or MXE. This component is unique to MDMA in that it almost always comes about in the form of self-affirmation and a personal appreciation for one’s self as well as others.
- Unity and interconnectedness - Experiences of lower-level unity and interconnectedness are commonly produced by MDMA. This component most consistently manifests itself within large crowds at raves and musical events in the form of "becoming one with the crowd." Music is said to consistently intensify this effect as well.
After effects 
-
The effects which occur during the offset of an entactogen or stimulant experience generally feel negative and uncomfortable in comparison to the effects which occurred during its peak. This is often referred to as a "come down" and is thought to occur because of neurotransmitter depletion. Its effects commonly include:
- Anxiety
- Appetite suppression
- Brain zaps
- Cognitive fatigue
- Depression
- Derealization
- Dream suppression or Dream potentiation - Although this substance have been known to suppress dreaming, some users note extremely strange and sometimes scary dreams for several nights after taking large doses of MDMA.
- Sleep paralysis - Some users report a higher incidence of experiencing sleep paralysis after consuming MDMA.
- Irritability
- Motivation suppression
- Thought deceleration
- Thought disorganization
- Suicidal ideation
- Wakefulness
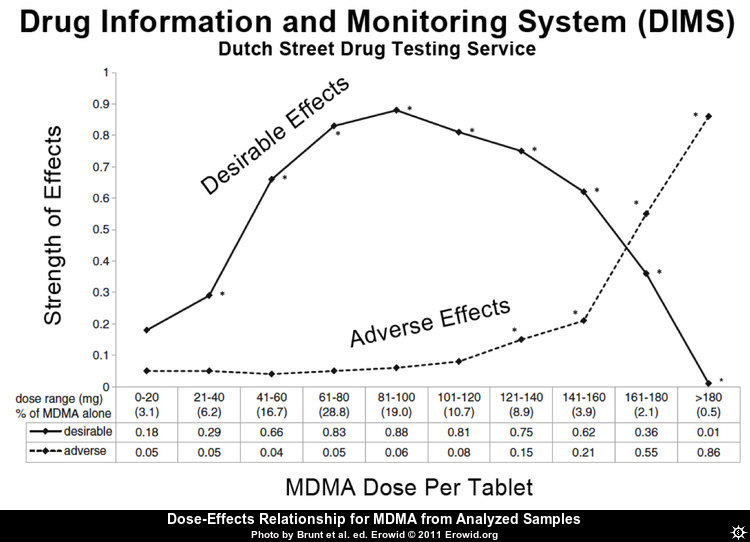
Dose-effects relationship from analyzed samples
The optimal single oral dose for an average user may be around 90 mg going by this diagram. Note that the percentages at the bottom are too outdated to guide in estimating substance content in current pills: The average concentration of MDMA in ecstasy pills, tested in a drug checking program in Zurich, doubled between 2010 and 2018. The percentage of pills containing more than 120 mg MDMA rose from 4% to 73%. In the same period, the rate of pills containing other psychoactive compounds dropped from 53% to 7%.[44]
Experience reports
Anecdotal reports which describe the effects of this compound within our experience index include:
- Experience:0.75g MDMA - Possibly some MDA through metabolisation?
- Experience:150mg MDMA + 20mg 2C-B - I designed it this way myself
- Experience:250mg MDA / 250mg MDMA - unnecessarily large dosage
- Experience:250mg MDMA (oral) - Pareidolia & paranoia
- Experience:450mg MDMA - Quarter consumption through whole night
- Experience:Cannabis, Ecstasy (3 brownies, 1 pill, Oral) My happy friends Shadow People
- Experience:MDMA (1/2 tab, oral) - My first time ever being high
- Experience:MDMA (100 mg) + Cannabis - Trip Report
- Experience:MDMA (750mg, Oral) - Finally Free
- Experience:MDMA (80mg, rectal) - Comments on rectal bioavailability
- Experience:MDMA or MDA, 580mg, Oral
- Experience:Nightmare flipping
Additional experience reports can be found here:
Names and forms
Names
Since the 1980s, MDMA has become widely known as "Ecstasy" (shortened to "E", "X", or "XTC"), usually referring to its street forms as illicitly pressed pills or tablets.[46] The American term "Molly" and the British equivalent term "Mandy" originally referred to crystal or powder MDMA that was purported to be of high purity and free of adulteration.[47] However, it has since evolved into a generic street term for any number of euphoric stimulants that are sold in powder or crystal form.[citation needed]
Forms
MDMA can be found in the following forms:
Crystals
Crystals or powder (commonly called Molly) is a white to brownish substance which can be dissolved, crushed, put into gel capsules or edible paper ("parachutes"). It can be administered orally, sublingually, buccally or via insufflation ("snorting" or "sniffing").
Pills
Pills are the most common form in which MDMA is sold, and are commonly referred to as Ecstasy. They often contain other substances or adulterants that range from anything from MDA, MDEA, amphetamine, methamphetamine, caffeine, 2C-B or mCPP to synthesis by-products such as MDP2P, MDDM or 2C-H. They can also contain an array random substances such as research chemicals, prescription drugs, over-the-counter drugs, poisons or nothing at all. It is strongly recommended to take harm reduction measures such as using a reagent testing kit when ingesting unknown pills.
The average concentration of MDMA in ecstasy pills, tested in a drug checking program in Zurich, doubled between 2010 and 2018. The percentage of pills containing more than 120 mg MDMA rose from 4% to 73%. In the same period, the rate of pills containing other psychoactive compounds dropped from 53% to 7%.[50]
Research
MDMA-assisted psychotherapy
Dosages
Common dosages for MDMA-assisted therapy:
- 1.1-1.7 mg/kg, or 80-120 mg for a 70 kg person.
- Kilo body weight + 50 in milligrams
MDMA-assisted psychotherapy for PTSD
In 2011, a pilot study on 20 patients demonstrated promising results in the treatment of post-traumatic stress disorder (PTSD). After two or three MDMA-assisted psychotherapy sessions, 83% of the patients no longer met the criteria for PTSD, compared to only 25% in the control group where MDMA was replaced with a placebo. The results sustained at two and twelve months after the treatment. The MDMA and placebo group both received non-drug psychotherapy before and after the sessions. In the study, a dose of 125mg MDMA plus a 62.5mg supplemental dose after 2 hours have been administered.[51] After completion of the study, the patients from the placebo group also received MDMA-assisted psychotherapy, and a long-term follow-up study of 19 patients published in 2013 shows that even after three years the positive results maintained.[51]
In 2017, the FDA granted MDMA a breakthrough therapy designation for PTSD, meaning if studies show promise, a review for potential medical use could occur more quickly.[52] Phase 3 clinical trials to look at effectiveness and safety have already begun, and are expected to be completed in 2021, meaning the FDA could approve treatment as early as 2022.[53] [54]
R-MDMA
MDMA is typically produced and consumed in its racemic form (known as SR-MDMA) which consists of equal parts S-MDMA and R-MDMA. A 2017 study found that high doses of R-MDMA administered in mice increased prosocial behavior and facilitated fear-extinction learning but did not produce hyperthermia or signs of neurotoxicity. This is thought to owe itself to the lower dopamine release R-MDMA displays relative to SR-MDMA. This result suggests that R-MDMA may be a safer and more viable therapeutic than racemic MDMA.[55] However, more research is needed to validate this finding.
Reagent results
Exposing compounds to the reagents gives a colour change which is indicative of the compound under test.
| Marquis | Mecke | Mandelin | Liebermann | Froehde | Gallic | |
|---|---|---|---|---|---|---|
| Purple - Black | Green - Blue / Black | Purple / Blue - Black | Intense brown - Black | Yellow/green - Dark blue | Green to brown | |
| Robadope | Ehrlich | Hofmann | Simon’s | Zimmermann | Scott | Folin |
| No reaction | No reaction | No reaction | Dark blue | No reaction | No reaction | Orange |
Toxicity and harm potential
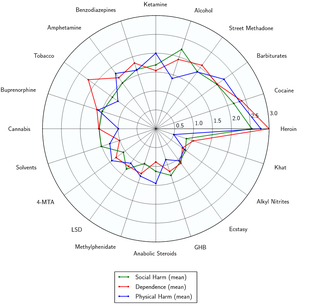
The short-term physical health risks of MDMA consumption include dehydration, bruxism, insomnia, hyperthermia,[57][58] and hyponatremia.[59] MDMA generally does not cause any serious or life threatening effects by itself unless it is associated with other extraneous factors such as exposure to prolonged high ambient temperature and humidity, prolonged physical activities, poor intake of water and lack of acclimatization.[60]
Continuous activity without sufficient rest or rehydration may cause the user's body temperature to rise to dangerous levels, and loss of fluid via excessive sweating puts the body at further risk as the stimulating and euphoric qualities of MDMA may cause the user to become oblivious to their physical condition.
Diuretics such as alcohol may exacerbate these risks further due to causing excessive amounts of dehydration. Users are advised to pay close attention to their water intake, drinking neither too much nor too little, and to take care not to overexert themselves to avoid heat-stroke, which can be fatal.
Toxic dose
The exact toxic dosage is unknown, but considered to be far greater than its effective dose.[citation needed]
Water intoxication and electrolyte imbalance
Water intoxication symptoms typically show up when a person consumes more than about 3-4 liters of water within an hour.[61][62] Also, an important cause of death following MDMA use is hyponatremia, low blood sodium levels as a result of drinking too much water.
Ecstasy users have reported that xerostomia (dry mouth) is common.[63][64] The excessive drinking observed in case reports of MDMA use may be caused by hyperpyrexia, a change in the primary drive to drink, and exposure to harm reduction messages that emphasize the need to drink water.[65] Higher doses of MDMA result in an overall difficulty when it comes to urination.[66] Urinary retention is caused by MDMA’s promotion of the release of anti-diuretic hormone (ADH); ADH is responsible for regulating urination. This effect can be lessened by relaxing, but can also be relieved by placing a hot flannel over the genitals to encourage blood flow.[67][68] However, MDMA causes water retention and dilution of electrolytes. Consequently, overhydration has caused death from water intoxication.[69] It was a main cause of the Death of Leah Betts who consumed an excstasy tablet followed by approximately 7 litres (1.8 US gal) of water in a 90 minute period, that resulted in water intoxication and hyponatremia, which in turn led to serious swelling of the brain, irreparably damaging it.
Users may experience signs of dehydration such as dry mouth and sweating while dancing or in a hot environment.
Water intoxication and electrolyte imbalance prevention
It is advised that users have hydration available, drink to thirst and never over-drink.[citation needed]
- Avoid consuming more than 3 liters of water within a few hours.
- Hyponatremia is preventable by drinking fluid containing sodium, such as that contained in sports drinks (typically ~20mM NaCl).
Neurotoxicity
The neurotoxicity of MDMA use is the subject of considerable debate. Scientific study has resulted in the general agreement that, although it is physically safe to try in a responsible context, the administration of repeated or high dosages of MDMA is most certainly neurotoxic in some form.
Administration of MDMA causes subsequent down-regulation of serotonin reuptake transporters in the brain. The rate at which the brain recovers from serotonergic changes is unclear. One study demonstrated lasting serotonergic changes in some animals exposed to MDMA.[70] Other studies have suggested that the brain may recover from serotonergic damage.[71][72][73]
It is thought that MDMA's metabolites play a large role in the uncertain levels of neurotoxicity. For example, a metabolite of MDMA called alpha-Methyldopamine (α-Me-DA, which is known to be toxic to dopamine neurons[74][18]Miller, R. T., Lau, S. S., Monks, T. J. (4 April 1997). "2,5-Bis-(glutathion-S-yl)-alpha-methyldopamine, a putative metabolite of (+/-)-3,4-methylenedioxyamphetamine, decreases brain serotonin concentrations". European Journal of Pharmacology. 323 (2–3): 173–180. doi:10.1016/s0014-2999(97)00044-7. ISSN 0014-2999. </ref>) was thought believed to be involved in the toxicity of MDMA to serotonin receptors.
However, one study found this to not be the case as direct administration of α-Me-DA did not cause neurotoxicity.[18] Additionally, MDMA injected directly into the brain was found to not be toxic, implying a metabolite is responsible for the toxicity when MDMA is administered via insufflation or oral consumption.[18]
This study found that although α-Me-DA is involved, it is a further metabolite of α-Me-DA involving glutathione that is primarily responsible for the selective damage to 5-HT receptors triggered by MDMA/MDA.[18]This metabolite forms in higher concentrations when core temperature is elevated. It is taken up into serotonin receptors by its transporters and metabolized by MAO-B into a reactive oxygen species which can cause neurological damage.[18][75]
Retracted article on dopaminergic neurotoxicity of MDMA
“Severe dopaminergic neurotoxicity in primates after a common recreational dose regimen of MDMA”[76] is an article by George A. Ricaurte which was published in September 2002 in the peer-reviewed journal Science, one of the world's top academic journals. It was later retracted; instead of using MDMA, methamphetamine had been used in the test.[77]
Cardiotoxicity
Long-term heavy use of MDMA has been shown to be cardiotoxic and may lead to valvulopathy (heart valve damage) through its actions on the 5-HT2B receptor.[78][75]
In one study, 28% of long-term users (2-3 doses per week for a mean of 6 years, mean of age 24.3 years) had developed clinically evident valvular heart disease.[79]
It is strongly recommended that one use harm reduction practices when using this substance.
Dependence and abuse potential
As with other stimulants, the chronic use of MDMA can be considered moderately addictive with a high potential for abuse and is capable of causing psychological dependence among certain users. When addiction has developed, cravings and withdrawal effects may occur if one suddenly stops their usage.
Tolerance to many of the effects of MDMA develops with prolonged and repeated use. This results in users having to administer increasingly larger doses to achieve the same effects.
Upon a single administration, it takes about 1 month for the tolerance to be reduced to half and 2.5 months to be back at baseline (in the absence of further consumption).
MDMA exhibits cross-tolerance with all dopaminergic and serotonergic stimulants, meaning that after the consumption of MDMA all stimulants will have a reduced effect.
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- 25x-NBOMe - Due to the highly unpredictable and physically straining effects of 25x-NBOMe, combinations with MDMA are strongly discouraged.
- Protease Inhibitors - Certain HIV medications like Ritonavir or Cobicistat contain protease inhibitors, that cause the liver to process MDMA slower resulting in longer and stronger effects, which causes more harm to the liver and might cause overdosing. A lower dose is recommended. [80]
- 5-MeO-xxT - 5-MeO tryptamines are considered to be unpredictable and should be mixed with MDMA with care.
- Alcohol - Both MDMA and alcohol cause dehydration and bodily strain. Approach this combination with caution, moderation and sufficient hydration. More than a small amount of alcohol will dull the euphoria of MDMA.
- Cocaine - Cocaine blocks some of the desirable effects of MDMA while increasing the risk of heart attack.[citation needed]
- DOx - The combined stimulating effects of DOx and MDMA can become overbearing, particularly during the come-up phase. Additionally, coming down on the MDMA while the DOx is still active can produce significant anxiety and bodily discomfort.
- GHB/GBL - Large amounts of GHB/GBL may overwhelm the effects of MDMA on the comedown and place the user at risk of sudden loss of consciousness.
- MXE - There have been reports of concerning serotonergic interactions when the two are taken at the same time, but MXE taken to the end of an MDMA experience does not appear to cause the same issues.
- PCP - PCP with MDMA may increase the risk of excessive stimulation, mania, and psychosis.
- Tramadol - Tramadol is well-documented to lower the seizure threshold[81] and this risk is especially elevated when tramadol is taken with MDMA.
Serotonin syndrome risk
Combinations with the following substances can lead to dangerously high serotonin levels. Serotonin syndrome requires immediate medical attention and can be fatal if left untreated.
- MAOIs such as syrian rue, banisteriopsis caapi, phenelzine, selegiline, and moclobemide[82] - MAO-B inhibitors can increase the potency and duration of phenethylamines unpredictably. MAO-A inhibitors with MDMA will lead to hypertensive crises.
- SSRIs and SNRIs may dampen the the desired psychological effects of MDMA while retaining the same level of undesirable physiological side-effects.[83][84]
- Serotonin releasers such as MDMA, 4-FA, methamphetamine, methylone and αMT
- AMT
- 2C-T-x
- DXM
- 5-HTP - 5-HTP is a supplement that acts as a precursor for serotonin. It is sometimes recommended to be used after MDMA experiences to try to restore depleted serotonin reserves. However, taking 5-HTP shortly before or with MDMA may cause excessive serotonin levels in the brain, which can lead to serotonin syndrome.[85] As a result, it is advised to wait until the day after the MDMA has been used before consuming 5-HTP.
Legal status
Internationally, MDMA was added to the UN Convention on Psychotropic Substances as a Schedule I controlled substance in February 1986.[86]
- Australia: MDMA is a Schedule 8 (Controlled Drugs) drug in Australia, starting July 1st 2023. [87]
- Austria: MDMA is illegal to possess, produce and sell under the SMG (Suchtmittelgesetz Österreich).[88]
- Belgium: MDMA is illegal to possess, produce and sell in Belgium.[89]
- Brazil: MDMA is illegal to possess, produce and sell under Portaria SVS/MS nº 344.[90]
- Canada: MDMA is a Schedule I drug in Canada.[91]
- Denmark: MDMA is illegal to possess, produce and sell in Denmark.[92]
- Egypt: MDMA is a Schedule III drug in Egypt.[citation needed]
- Finland: MDMA is illegal to possess, produce and sell in Finland.[citation needed]
- Germany: MDMA is controlled under Anlage I BtMG (Narcotics Act, Schedule I)[93] as of August 1, 1986.[94] It is illegal to manufacture, possess, import, export, buy, sell, procure or dispense it without a license.[95]
- Latvia: MDMA is a Schedule I drug in Latvia.[96]
- Luxembourg: MDMA is a prohibited substance.[97]
- The Netherlands: MDMA is illegal to possess, produce and sell in the Netherlands.[98]
- New Zealand: MDMA is a Class B1 drug in New Zealand.[99]
- Norway: MDMA is illegal to possess, produce and sell in Norway.[citation needed]
- Portugal: MDMA is illegal to produce, sell or trade in Portugal. However, since 2001, individuals found in possession of small quantities (up to 1 gram) are considered sick individuals instead of criminals. The drugs are confiscated and the suspects may be forced to attend a dissuasion session at the nearest CDT (Commission for the Dissuasion of Drug Addiction) or pay a fine, in most cases.[100]
- Russia: MDMA is classified as a Schedule I prohibited substance.[101]
- Sweden: MDMA is illegal to possess, produce and sell in Sweden.[citation needed]
- Switzerland: MDMA is a controlled substance specifically named under Verzeichnis D.[102]
- United Kingdom: MDMA is a Class A drug in the UK.[103]
- United States: MDMA is classified as a Schedule I drug under the Controlled Substance Act. This means it is illegal to manufacture, buy, possess, process, or distribute without a license from the Drug Enforcement Administration (DEA).[104]
- Czech Republic: MDMA is a Schedule I controlled substance.[105]
See also
External links
Harm reduction
- Calculators
- EcstasyData
- Pill Reports
- RollSafe
- MDMA Wiki (archived)
References
- ↑ 1.0 1.1 1.2 Freudenmann, Roland W.; Öxler, Florian; Bernschneider-Reif, Sabine (2006). "The origin of MDMA (ecstasy) revisited:the true story reconstructed from the original documents". Addiction. 101 (9): 1241–1245. doi:10.1111/j.1360-0443.2006.01511.x. ISSN 1360-0443.
- ↑ 2.0 2.1 2.2 Shulgin, Alexander; Shulgin, Ann (1991). "Chapter 12". PiHKAL: A Chemical Love Story. Part 1. Transform Press. pp. 66–74. ISBN 0963009605.
- ↑ Merck and Ecstasy / MDMA
- ↑ The Global Drug Survey 2014 findings
- ↑ World Health Organization, ed. (2004). Neuroscience of psychoactive substance use and dependence. World Health Organization. ISBN 9789241562355.
- ↑ Multidisciplinary Association for Psychedelic Studies (2022), A Placebo-controlled, Randomized, Blinded, Dose Finding Phase 2 Pilot Safety Study of MDMA-assisted Therapy for Social Anxiety in Autistic Adults, clinicaltrials.gov
- ↑ MDMA-Assisted Therapy for Anxiety Associated with Life-Threatening Illness (MDA-1)
- ↑ Meyer, J. S. (21 November 2013). "3,4-methylenedioxymethamphetamine (MDMA): current perspectives". Substance Abuse and Rehabilitation. 4: 83–99. doi:10.2147/SAR.S37258.
- ↑ Parrott, A. C. (March 2014). "The potential dangers of using MDMA for psychotherapy". Journal of Psychoactive Drugs. 46 (1): 37–43. doi:10.1080/02791072.2014.873690. ISSN 0279-1072.
- ↑ Meyer, J. S. (2013). "3,4-methylenedioxymethamphetamine (MDMA): current perspectives". Substance Abuse and Rehabilitation. 4: 83–99. doi:10.2147/SAR.S37258. ISSN 1179-8467.
- ↑ Greene, S. L., Kerr, F., Braitberg, G. (October 2008). "Review article: amphetamines and related drugs of abuse". Emergency medicine Australasia: EMA. 20 (5): 391–402. doi:10.1111/j.1742-6723.2008.01114.x. ISSN 1742-6723.
- ↑ Nestler, E. J., Hyman, S. E., Malenka, R. C. (2009). Molecular neuropharmacology: a foundation for clinical neuroscience (2nd ed ed.). McGraw-Hill Medical. ISBN 9780071481274.
- ↑ Gouzoulis-Mayfrank, E., Daumann, J. (2009). "Neurotoxicity of drugs of abuse--the case of methylenedioxyamphetamines (MDMA, ecstasy), and amphetamines". Dialogues in Clinical Neuroscience. 11 (3): 305–317. ISSN 1294-8322.
- ↑ 14.0 14.1 Karch, Steven (2011). "A Historical Review of MDMA". The Open Forensic Science Journal. 4: 20–24. doi:10.2174/1874402801104010020. ISSN 1874-4028.
- ↑ 15.0 15.1 Sessa, B. (2017). The Psychedelic Renaissance: Reassessing the Role of Psychedelic Drugs in 21st Century Psychiatry and Society. Muswell Hill Press. ISBN 9781908995278.
- ↑ Young, Francis L. (May 22, 1968). "In The Matter Of MDMA Scheduling: Opinion And Recommended Ruling, Findings Of Fact, Conclusions Of Law And Decision Of Administrative Law Judge On Issues Two Through Seven" (PDF). maps.org. Multidisciplinary Association for Psychedelic Studies. Retrieved November 14, 2019.
- ↑ "3,4-Methylenedioxymethamphetamine". Hazardous Substances Data Bank. National Library of Medicine. 28 August 2008. Retrieved 22 August 2014.
- ↑ 18.00 18.01 18.02 18.03 18.04 18.05 18.06 18.07 18.08 18.09 18.10 Miller, G. M. (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity: TAAR1 regulation of monoaminergic activity". Journal of Neurochemistry. 116 (2): 164–176. doi:10.1111/j.1471-4159.2010.07109.x. ISSN 0022-3042.
- ↑ 19.0 19.1 19.2 Eiden, L. E., Weihe, E. (January 2011). "VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse: VMAT2 and addiction". Annals of the New York Academy of Sciences. 1216 (1): 86–98. doi:10.1111/j.1749-6632.2010.05906.x. ISSN 0077-8923.
- ↑ Fitzgerald, J. L., Reid, J. J. (November 1990). "Effects of methylenedioxymethamphetamine on the release of monoamines from rat brain slices". European Journal of Pharmacology. 191 (2): 217–220. doi:10.1016/0014-2999(90)94150-V. ISSN 0014-2999.
- ↑ Eiden, L. E., Weihe, E. (January 2011). "VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse: VMAT2 and addiction". Annals of the New York Academy of Sciences. 1216 (1): 86–98. doi:10.1111/j.1749-6632.2010.05906.x. ISSN 0077-8923.
- ↑ Bogen, I. L., Haug, K. H., Myhre, O., Fonnum, F. (September 2003). "Short- and long-term effects of MDMA ("ecstasy") on synaptosomal and vesicular uptake of neurotransmitters in vitro and ex vivo". Neurochemistry International. 43 (4–5): 393–400. doi:10.1016/S0197-0186(03)00027-5. ISSN 0197-0186.
- ↑ Nelson, L., Goldfrank, L. R., eds. (2011). Goldfrank’s toxicologic emergencies (9th ed ed.). McGraw-Hill Medical. ISBN 9780071605939.
- ↑ Battaglia, G., Brooks, B. P., Kulsakdinun, C., De Souza, E. B. (April 1988). "Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites". European Journal of Pharmacology. 149 (1–2): 159–163. doi:10.1016/0014-2999(88)90056-8. ISSN 0014-2999.
- ↑ Lyon, RobertA., Glennon, RichardA., Titeler, M. (April 1986). "3,4-Methylenedioxymethamphetamine (MDMA): Stereoselective interactions at brain 5-HT1 and 5-HT2 receptors". Psychopharmacology. 88 (4). doi:10.1007/BF00178519. ISSN 0033-3158.
- ↑ Nash, J. F., Roth, B. L., Brodkin, J. D., Nichols, D. E., Gudelsky, G. A. (August 1994). "Effect of the R(−) and S(+) isomers of MDA and MDMA on phosphotidyl inositol turnover in cultured cells expressing 5-HT2A or 5-HT2C receptors". Neuroscience Letters. 177 (1–2): 111–115. doi:10.1016/0304-3940(94)90057-4. ISSN 0304-3940.
- ↑ Setola, V., Hufeisen, S. J., Grande-Allen, K. J., Vesely, I., Glennon, R. A., Blough, B., Rothman, R. B., Roth, B. L. (June 2003). "3,4-Methylenedioxymethamphetamine (MDMA, "Ecstasy") Induces Fenfluramine-Like Proliferative Actions on Human Cardiac Valvular Interstitial Cells in Vitro". Molecular Pharmacology. 63 (6): 1223–1229. doi:10.1124/mol.63.6.1223. ISSN 0026-895X.
- ↑ Betzler, F., Viohl, L., Romanczuk-Seiferth, N. (January 2017). Foxe, J., ed. "Decision-making in chronic ecstasy users: a systematic review". European Journal of Neuroscience. 45 (1): 34–44. doi:10.1111/ejn.13480. ISSN 0953-816X.
- ↑ Matsumoto, R. R. (July 2009). "Targeting sigma receptors: novel medication development for drug abuse and addiction". Expert Review of Clinical Pharmacology. 2 (4): 351–358. doi:10.1586/ecp.09.18. ISSN 1751-2433.
- ↑ 30.0 30.1 Kalant, H. (2 October 2001). "The pharmacology and toxicology of "ecstasy" (MDMA) and related drugs". CMAJ: Canadian Medical Association Journal. 165 (7): 917–928. ISSN 0820-3946.
- ↑ Bexis, S., Docherty, J. R. (April 2006). "Effects of MDMA, MDA and MDEA on blood pressure, heart rate, locomotor activity and body temperature in the rat involve α -adrenoceptors: MDMA on rat vascular and temperature responses". British Journal of Pharmacology. 147 (8): 926–934. doi:10.1038/sj.bjp.0706688. ISSN 0007-1188.
- ↑ Liechti, M. E. (31 October 2014). "Effects of MDMA on body temperature in humans". Temperature: Multidisciplinary Biomedical Journal. 1 (3): 192–200. doi:10.4161/23328940.2014.955433. ISSN 2332-8940.
- ↑ 3,4-Methylenedioxymethamphetamine (MDMA, Ecstasy) and Driving Impairment
- ↑ http://www.idmu.co.uk/therapeutic-uses-of-ecstasy.htm
- ↑ Greer, G. R., Tolbert, R. (December 1998). "A method of conducting therapeutic sessions with MDMA". Journal of Psychoactive Drugs. 30 (4): 371–379. doi:10.1080/02791072.1998.10399713. ISSN 0279-1072.
- ↑ shirelle@maps.org (1995), A Dose/Response Human Pilot Study – Safety and Efficacy of MDMA in Modification of Physical Pain and Psychological Distress in End-Stage Cancer Patients, retrieved 2 August 2022
- ↑ Buffum, John; Moser, Charles (October 1986). "MDMA and Human Sexual Function". Journal of Psychoactive Drugs. 18 (4): 355–359. doi:10.1080/02791072.1986.10472369. ISSN 0279-1072. PMID 2880951.
- ↑ Zemishlany, Z.; Aizenberg, D.; Weizman, A. (March 2001). "Subjective effects of MDMA ('Ecstasy') on human sexual function". European Psychiatry. 16 (2): 127–130. doi:10.1016/s0924-9338(01)00551-x. ISSN 0924-9338. PMID 11311179. Unknown parameter
|s2cid=ignored (help) - ↑ Dinis-Oliveira, R. J., Caldas, I., Carvalho, F., Magalhães, T. (1 October 2010). "Bruxism after 3,4-methylenedioxymethamphetamine (ecstasy) abuse". Clinical Toxicology. 48 (8): 863–864. doi:10.3109/15563650.2010.489903. ISSN 1556-3650.
- ↑ Urban Dictionary: gurning
- ↑ Parrott, Andy C.; Milani, Raffaella M.; Parmar, Rishee; Turner, John J. (2001-09-11). "Recreational ecstasy/MDMA and other drug users from the UK and Italy: psychiatric symptoms and psychobiological problems". Psychopharmacology. 159 (1): 77–82. doi:10.1007/s002130100897. ISSN 0033-3158. PMID 11797073. Unknown parameter
|s2cid=ignored (help) - ↑ Passie, Torsten; Hartmann, Uwe; Schneider, Udo; Emrich, Hinderk M.; Krüger, Tillmann H.C. (January 2005). "Ecstasy (MDMA) mimics the post-orgasmic state: Impairment of sexual drive and function during acute MDMA-effects may be due to increased prolactin secretion". Medical Hypotheses. 64 (5): 899–903. doi:10.1016/j.mehy.2004.11.044. ISSN 0306-9877. PMID 15780482.
- ↑ Topp, Libby; Hando, Julie; Dillon, Paul; Roche, Ann; Solowij, Nadia (June 1999). "Ecstasy use in Australia: patterns of use and associated harm". Drug and Alcohol Dependence. 55 (1–2): 105–115. doi:10.1016/s0376-8716(99)00002-2. ISSN 0376-8716. PMID 10402155.
- ↑ "MDMA Auswertung 2018" [MDMA Evaluation 2018] (PDF). saferparty.ch (in German). Sozialdepartement Zürich. 2018. Retrieved January 18, 2020.
- ↑ Effects Relationship for MDMA from Analyzed Samples (DIMS 2011)
- ↑ Green, A. R., Mechan, A. O., Elliott, J. M., O’Shea, E., Colado, M. I. (1 September 2003). "The Pharmacology and Clinical Pharmacology of 3,4-Methylenedioxymethamphetamine (MDMA, "Ecstasy")". Pharmacological Reviews. 55 (3): 463–508. doi:10.1124/pr.55.3.3. ISSN 0031-6997.
- ↑ Kahn, D. E., Ferraro, N., Benveniste, R. J. (15 December 2012). "3 cases of primary intracranial hemorrhage associated with "Molly", a purified form of 3,4-methylenedioxymethamphetamine (MDMA)". Journal of the Neurological Sciences. 323 (1): 257–260. doi:10.1016/j.jns.2012.08.031. ISSN 0022-510X.
- ↑ Shulgin Index, p811
- ↑ http://www.ecstasy.org/testing/pma.html
- ↑ "MDMA Auswertung 2018" [MDMA Evaluation 2018] (PDF). saferparty.ch (in German). Sozialdepartement Zürich. 2018. Retrieved January 18, 2020.
- ↑ 51.0 51.1 Mithoefer, M. C., Wagner, M. T., Mithoefer, A. T., Jerome, L., Martin, S. F., Yazar-Klosinski, B., Michel, Y., Brewerton, T. D., Doblin, R. (January 2013). "Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: a prospective long-term follow-up study". Journal of Psychopharmacology. 27 (1): 28–39. doi:10.1177/0269881112456611. ISSN 0269-8811.
- ↑ Wan, W. (2017), Ecstasy could be ‘breakthrough’ therapy for soldiers, others suffering from PTSD, retrieved August 29, 2017
- ↑ Feduccia, A. A., Holland, J., Mithoefer, M. C. (February 2018). "Progress and promise for the MDMA drug development program". Psychopharmacology. 235 (2): 561–571. doi:10.1007/s00213-017-4779-2. ISSN 1432-2072.
- ↑ Inverse: MDMA Steps Closer to FDA Approval as a Drug, but Now it Needs to Leap, 2016
- ↑ Curry, D. W., Young, M. B., Tran, A. N., Daoud, G. E., Howell, L. L. (January 2018). "Separating the agony from ecstasy: R(–)-3,4-methylenedioxymethamphetamine has prosocial and therapeutic-like effects without signs of neurotoxicity in mice". Neuropharmacology. 128: 196–206. doi:10.1016/j.neuropharm.2017.10.003. ISSN 0028-3908.
- ↑ Nutt, D., King, L. A., Saulsbury, W., Blakemore, C. (March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". The Lancet. 369 (9566): 1047–1053. doi:10.1016/S0140-6736(07)60464-4. ISSN 0140-6736.
- ↑ Nimmo, S. M., Kennedy, B. W., Tullett, W. M., Blyth, A. S., Dougall, J. R. (October 1993). "Drug-induced hyperthermia". Anaesthesia. 48 (10): 892–895. doi:10.1111/j.1365-2044.1993.tb07423.x. ISSN 0003-2409.
- ↑ Malberg, J. E., Seiden, L. S. (1 July 1998). "Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat". The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 18 (13): 5086–5094. ISSN 0270-6474.
- ↑ Wolff, K., Tsapakis, E. M., Winstock, A. R., Hartley, D., Holt, D., Forsling, M. L., Aitchison, K. J. (May 2006). "Vasopressin and oxytocin secretion in response to the consumption of ecstasy in a clubbing population". Journal of Psychopharmacology. 20 (3): 400–410. doi:10.1177/0269881106061514. ISSN 0269-8811.
- ↑ Nadesan, K., Kumari, C., Afiq, M. (1 August 2017). "Dancing to death: A case of heat stroke". Journal of Forensic and Legal Medicine. 50: 1–5. doi:10.1016/j.jflm.2017.05.008. ISSN 1752-928X.
- ↑ https://www.healthline.com/health/how-much-water-can-kill-you
- ↑ https://www.medicalnewstoday.com/articles/318619
- ↑ https://pubmed.ncbi.nlm.nih.gov/18268544/
- ↑ https://pubmed.ncbi.nlm.nih.gov/10403088/
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4923534/
- ↑ https://www.sciencedirect.com/science/article/abs/pii/S0736467903003433
- ↑ Bora, F., Yılmaz, F., Bora, T. (November 2016). "Ecstasy (MDMA) and its effects on kidneys and their treatment: a review". Iranian Journal of Basic Medical Sciences. 19 (11): 1151–1158. ISSN 2008-3866.
- ↑ Inman, D. S., Greene, D. (January 2003). "'The agony and the ecstasy': acute urinary retention after MDMA abuse". BJU International. 91 (1): 123–123. doi:10.1046/j.1464-410X.2003.04018.x. ISSN 1464-4096.
- ↑ Brvar, M., Kozelj, G., Osredkar, J., Mozina, M., Gricar, M., Bunc, M. (October 2004). "Polydipsia as another mechanism of hyponatremia after "ecstasy" (3,4 methyldioxymethamphetamine) ingestion". European Journal of Emergency Medicine: Official Journal of the European Society for Emergency Medicine. 11 (5): 302–304. doi:10.1097/00063110-200410000-00014. ISSN 0969-9546.
- ↑ Fischer, C., Hatzidimitriou, G., Wlos, J., Katz, J., Ricaurte, G. (August 1995). "Reorganization of ascending 5-HT axon projections in animals previously exposed to the recreational drug (+/-)3,4-methylenedioxymethamphetamine (MDMA, "ecstasy")". The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 15 (8): 5476–5485. ISSN 0270-6474.
- ↑ Scheffel, U., Szabo, Z., Mathews, W. B., Finley, P. A., Dannals, R. F., Ravert, H. T., Szabo, K., Yuan, J., Ricaurte, G. A. (June 1998). <183::AID-SYN9>3.0.CO;2-3 "In vivo detection of short- and long-term MDMA neurotoxicity?a positron emission tomography study in the living baboon brain". Synapse. 29 (2): 183–192. doi:10.1002/(SICI)1098-2396(199806)29:2<183::AID-SYN9>3.0.CO;2-3. ISSN 0887-4476.
- ↑ Reneman, L., Lavalaye, J., Schmand, B., Wolff, F. A. de, Brink, W. van den, Heeten, G. J. den, Booij, J. (1 October 2001). "Cortical Serotonin Transporter Density and Verbal Memory in Individuals Who Stopped Using 3,4-Methylenedioxymethamphetamine (MDMA or "Ecstasy"): Preliminary Findings". Archives of General Psychiatry. 58 (10): 901. doi:10.1001/archpsyc.58.10.901. ISSN 0003-990X.
- ↑ Selvaraj, S., Hoshi, R., Bhagwagar, Z., Murthy, N. V., Hinz, R., Cowen, P., Curran, H. V., Grasby, P. (April 2009). "Brain serotonin transporter binding in former users of MDMA ('ecstasy')". British Journal of Psychiatry. 194 (4): 355–359. doi:10.1192/bjp.bp.108.050344. ISSN 0007-1250.
- ↑ Perfetti, X., O’Mathúna, B., Pizarro, N., Cuyàs, E., Khymenets, O., Almeida, B., Pellegrini, M., Pichini, S., Lau, S. S., Monks, T. J., Farré, M., Pascual, J. A., Joglar, J., Torre, R. de la (July 2009). "Neurotoxic thioether adducts of 3,4-methylenedioxymethamphetamine identified in human urine after ecstasy ingestion". Drug Metabolism and Disposition: The Biological Fate of Chemicals. 37 (7): 1448–1455. doi:10.1124/dmd.108.026393. ISSN 1521-009X.
- ↑ 75.0 75.1 Elangbam, C. S. (October 2010). "Drug-induced Valvulopathy: An Update". Toxicologic Pathology. 38 (6): 837–848. doi:10.1177/0192623310378027. ISSN 0192-6233.
- ↑ Ricaurte GA, Yuan J, Hatzidimitriou G, Cord BJ, McCann UD (September 2002). "Severe dopaminergic neurotoxicity in primates after a common recreational dose regimen of MDMA ("ecstasy")". Science. 297 (5590): 2260–3. Bibcode:2002Sci...297.2260R. doi:10.1126/science.1074501. PMID 12351788. Unknown parameter
|s2cid=ignored (help) - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedRicaurte-Retraction - ↑ Rothman, R. B., Baumann, M. H., Savage, J. E., Rauser, L., McBride, A., Hufeisen, S. J., Roth, B. L. (5 December 2000). "Evidence for Possible Involvement of 5-HT 2B Receptors in the Cardiac Valvulopathy Associated With Fenfluramine and Other Serotonergic Medications". Circulation. 102 (23): 2836–2841. doi:10.1161/01.CIR.102.23.2836. ISSN 0009-7322.
- ↑ Droogmans, S., Cosyns, B., D’haenen, H., Creeten, E., Weytjens, C., Franken, P. R., Scott, B., Schoors, D., Kemdem, A., Close, L., Vandenbossche, J.-L., Bechet, S., Van Camp, G. (1 November 2007). "Possible association between 3,4-methylenedioxymethamphetamine abuse and valvular heart disease". The American Journal of Cardiology. 100 (9): 1442–1445. doi:10.1016/j.amjcard.2007.06.045. ISSN 0002-9149.
- ↑ Harrington, Robert D.; Woodward, Jane A.; Hooton, Thomas M.; Horn, John R. (1999-10-11). "Life-Threatening Interactions Between HIV-1 Protease Inhibitors and the Illicit Drugs MDMA and γ-Hydroxybutyrate". Archives of Internal Medicine. American Medical Association (AMA). 159 (18): 2221. doi:10.1001/archinte.159.18.2221. ISSN 0003-9926.
- ↑ Talaie, H.; Panahandeh, R.; Fayaznouri, M. R.; Asadi, Z.; Abdollahi, M. (2009). "Dose-independent occurrence of seizure with tramadol". Journal of Medical Toxicology. 5 (2): 63–67. doi:10.1007/BF03161089. eISSN 1937-6995. ISSN 1556-9039. OCLC 163567183. PMC 3550327
 . PMID 19415589.
. PMID 19415589.
- ↑ Gillman, P. K. (October 2005). "Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity". British Journal of Anaesthesia. 95 (4): 434–441. doi:10.1093/bja/aei210. ISSN 0007-0912.
- ↑ Ewens, H. (2017), Everything You Need to Know About Mixing MDMA and Antidepressants
- ↑ Dobry, Y., Rice, T., Sher, L. (2013). "Ecstasy use and serotonin syndrome: a neglected danger to adolescents and young adults prescribed selective serotonin reuptake inhibitors". International Journal of Adolescent Medicine and Health. 25 (3): 193–199. doi:10.1515/ijamh-2013-0052. ISSN 0334-0139.
- ↑ Silins, E.; Copeland, J.; Dillon, P. (2007). "Qualitative Review of Serotonin Syndrome, Ecstasy (MDMA) and the use of Other Serotonergic Substances: Hierarchy of Risk". Australian and New Zealand Journal of Psychiatr. 41 (8): 649–655. doi:10.1080/00048670701449237. eISSN 1440-1614. ISSN 0004-8674. PMID 17620161.
- ↑ "Decision to place MDMA into Schedule I" (PDF). UNODC. Commission on Narcotic Drugs. 11 February 1986. Retrieved November 11, 2019.
- ↑ Australian MDMA and Psilocybin, new laws., 2023
- ↑ Suchtgiftverordnung, aktuelle Fassung
- ↑ EMCDDA Country legal profiles - Belgium | http://www.emcdda.europa.eu/html.cfm/index5174EN.html?pluginMethod=eldd.countryprofiles&country=BE
- ↑ List of controlled substances: Portaria SVS/MS nº 344 (Portuguese) | http://portal.anvisa.gov.br/lista-de-substancias-sujeitas-a-controle-especial
- ↑ Branch, L. S. (2022), Consolidated federal laws of canada, Controlled Drugs and Substances Act
- ↑ EMCDDA Country legal profiles - Denmark | http://www.emcdda.europa.eu/html.cfm/index5174EN.html?pluginMethod=eldd.countryprofiles&country=DK
- ↑ "Anlage I BtMG" (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 18, 2019.
- ↑ "Zweite Verordnung zur Änderung betäubungsmittelrechtlicher Vorschriften" (in German). Bundesanzeiger Verlag. Retrieved December 18, 2019.
- ↑ "§ 29 BtMG" (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 18, 2019.
- ↑ Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem (3,4-Metilēndioksifeniletānamīni)
- ↑ Règlement grand-ducal du 13 juin 1986 complétant l'annexe du règlement grand-ducal du 20 mars 1974 concernant certaines substances psychotropes. | http://legilux.public.lu/eli/etat/leg/rgd/1986/06/13/n2/jo
- ↑ Opiumwet | https://wetten.overheid.nl/BWBR0001941/2019-07-19&xid=17259,15700022,15700186,15700191,15700256,15700259,15700262,15700265,15700271,15700283&usg=ALkJrhg0a81esxOUix1UMvvAvbVALDP2-Q#BijlageI
- ↑ Misuse of Drugs Act 1975 No 116 (as at 01 July 2022), Public Act – New Zealand Legislation
- ↑ Greenwald, G. (2009), Drug Decriminalization in Portugal: Lessons for Creating Fair and Successful Drug Policies, Social Science Research Network
- ↑ Resolution of the Government of the Russian Federation | https://www.consultant.ru/cons/cgi/online.cgi?req=doc&base=LAW&n=314201&fld=134&dst=100034,0&rnd=0.41568319511755825#047741519652799347
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
- ↑ Misuse of Drugs Act 1971
- ↑ DEA / Drug Scheduling | https://www.dea.gov/druginfo/ds.shtml
- ↑ https://www.zakonyprolidi.cz/cs/2013-463#f5150333