Arylcyclohexylamines
This article is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
Arylcyclohexylamine refers to a class of compounds which typically produce dissociation, anesthesia and hallucinogenic effects.
Chemistry
Arylcyclohexylamines are comprised of an amine moiety and an aromatic ring attached to a cyclohexane ring.
Hydrogen atoms around the structure can be substituted for other functional groups to produce drugs of varying potency, affinity, efficacy and half-life.
Pharmacology
Arylcyclohexylamines are believed to produce their effects primarily by antagonism of NMDA receptors.[1]Antagonism of the NMDA receptor confers anesthetic, anticonvulsant, and dissociative effects. Contrary to popular belief, most arylcyclohexylamines do not significantly inhibit re-uptake of dopamine or agonise μ-opioid receptors.
| Compound | NMDA[3] | SERT[4] | NET[5] | Sigma1 | Sigma2 |
|---|---|---|---|---|---|
| Ketamine | 6.18±0.07 (659) | - | - | - | - |
| Phencyclidine (PCP) | 7.23±0.07 (59) | 5.65±0.05 (2234) | - | - | 6.82±0.09 (136) |
| Methoxetamine (MXE) | 6.59±0.06 (259) | 6.32±0.05 (481) | - | - | - |
| 4-MeO-PCP | 6.39±0.06 (404) | 6.07±0.05 (844) | 6.1±0.1 (713) | 6.5±0.1 (296) | 7.93±0.08 (143) |
| 3-MeO-PCP | 7.69±0.08 (20) | 6.7±0.1 (216) | - | 7.4±0.1 (42) | - |
| 3-MeO-PCE | 7.22±0.08 (61) | 6.9±0.06 (115) | - | 5.3±0.1 (4519) | 6.31±0.1 (525) |
List of substituted arylcyclohexylamines
| Compound | RN1 | RN2 | R2 | R3 | R4 | R2' | Structure |
|---|---|---|---|---|---|---|---|
| 2-BDCK | Methyl | H | Br | H | H | Ketone | 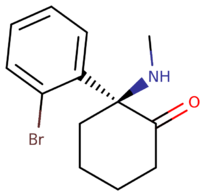
|
| 2-FDCK | Methyl | H | F | H | H | Ketone | |
| 2-TFMDCK | Methyl | H | CF3 | H | H | Ketone | |
| 3,4-MD-PCP | CH2CH2CH2- | CH2CH2- | H | OCH- | O- | H | 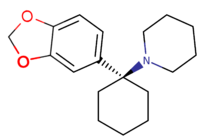
|
| O-PCE | Ethyl | H | H | H | H | Ketone | |
| Deschloroketamine
(DCK, O-PCM) |
Methyl | H | H | H | H | Ketone | |
| 3-Cl-PCP | CH2CH2CH2- | CH2CH2- | H | Cl | H | H | 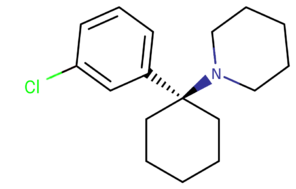
|
| 3-F-PCP | CH2CH2CH2- | CH2CH2- | H | F | H | H | 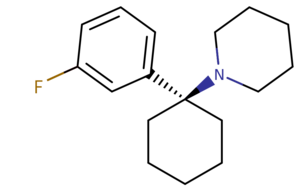
|
| 3-HO-PCE | Ethyl | H | H | Hydoxy | H | H | |
| 3-HO-PCP | CH2CH2CH2- | CH2CH2- | H | Hydroxy | H | H | |
| 3-Me-PCE | Ethyl | H | H | Methyl | H | H | 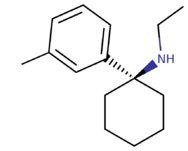
|
| 3-Me-PCP | CH2CH2CH2- | CH2CH2- | H | Methyl | H | H | 
|
| 3-Me-PCPy | CH2CH2- | CH2CH2- | H | Methyl | H | H | 
|
| 3-MeO-PCE | Ethyl | H | H | Methoxy | H | H | |
| 3-MeO-PCMo | CH2CH2O- | CH2CH2- | H | Methoxy | H | H | |
| 3-MeO-PCP | CH2CH2CH2- | CH2CH2- | H | Methoxy | H | H | |
| 4-MeO-PCP | CH2CH2CH2- | CH2CH2- | H | H | Methoxy | H | |
| Eticyclidine (PCE) | Ethyl | H | H | H | H | H | |
| HXE | Ethyl | H | H | Hydroxy | H | Ketone | |
| Phencyclidine (PCP) | CH2CH2CH2- | CH2CH2- | H | H | H | H | |
| Ketamine | Methyl | H | Cl | H | H | Ketone | |
| Methoxetamine | Ethyl | H | H | Methoxy | H | Ketone | |
| MXiPr | Isopropyl | H | H | Methoxy | H | Ketone |
Legal status
 |
This legality section is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
- United Kingdom - The arylcyclohexylamine family became illegal in the United Kingdom 2013.[citation needed]
See also
External links
References
- ↑ Ahmadi, A., Khalili, M., Hajikhani, R., Naserbakht, M. (1 April 2011). "New morpholine analogues of phencyclidine: Chemical synthesis and pain perception in rats". Pharmacology Biochemistry and Behavior. 98 (2): 227–233. doi:10.1016/j.pbb.2010.12.019. ISSN 0091-3057.
- ↑ Roth, B. L., Gibbons, S., Arunotayanun, W., Huang, X.-P., Setola, V., Treble, R., Iversen, L. (19 March 2013). "The Ketamine Analogue Methoxetamine and 3- and 4-Methoxy Analogues of Phencyclidine Are High Affinity and Selective Ligands for the Glutamate NMDA Receptor". PLOS ONE. 8 (3): e59334. doi:10.1371/journal.pone.0059334. ISSN 1932-6203.
- ↑ N-methyl-D-aspartate receptor
- ↑ Serotonin transporter
- ↑ Norepinephrine transporter